class6
advertisement

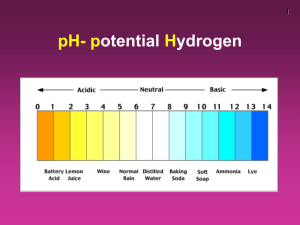
GES 430 Environmental Geochemistry Class 6 Acidity, Alkalinity + Buffering Or [H+] = ( 0.25 • (Ka1 • kCO2 • PCO2 √Ksp )^2 •Ka2 1/3 ) Plugging the numbers in we get a pH of 8.35 Waters will initially be acidic but once they come into contact with dolomite they will become basic. As your book shows calcite has a very similar effects. Interaction with carbonate minerals is one of the best ways of countering the effects of acid precipitation. If water is in contact with the atmosphere pH calculation known as open system calculations if not they are closed system calculations. For closed systems we often do not know PCO2 must have additional information. One assumption often used is the constant total carbon (CT) assumption see book page 67. Open systems Closed systems Acidity is a measure of the ability of a solution to donate H+. Example of “donation” of H+: What will happen OH- is added to a solution containing H3SiO4- ? H3SiO4- + OH- → H2SiO4-2 + H2O The solution has donated a H+ to the hydroxyl. Alkalinity is the capacity of a solution to accept H+. Example of “acceptance” of H+: What will happen H+ is added to a solution containing H3SiO4- ? H3SiO4- + H+ → H4SiO4 The solution has “accepted a H+ Quantitatively acidity (designated as CA) is defined as the sum of the concentration of all species that can donate H+ minus the concentration of OH-. Example: write an expression for the acidity of a solution that results from the dissolution of H4SiO4 in pure H2O? CA = [H4SiO4] + [H3SiO4-] + [H+] – [OH-] Note that we have ignored the very weak 3rd and 4th dissociation of H4SiO4 i.e. we have assumed that H2SiO4-2 → HSiO4-3 + H+ and HSiO4-3 → SiO4-4 + H+ are negligible. Quantitatively alkalinity (designated as CB) is defined as the sum of the concentration of all species that can accept H+ minus the concentration of H+. Example: write an expression for the alkalinity of a solution that results from the dissolution of H4SiO4 in pure H2O? CB = [H3SiO4-] + [H2SiO4-2] + [OH-] – [H+] Note that we have ignored the very weak 3rd and 4th dissociation of H4SiO4 i.e. we have assumed that H2SiO4-2 → HSiO4-3 + H+ and HSiO4-3 → SiO4-4 + H+ are negligible. Acidity and alkalinity are often given in milli-equivalent per liter (meq/L) = moles/L of the species • number of H+ that can be donated or accepted by that species. Thus in the examples given above: CA = [H4SiO4] + [H3SiO4-] + [H+] – [OH-] CA(meq/L) = 2 • [H4SiO4] + [H3SiO4-] + [H+] – [OH-] and CB = [H3SiO4-] + [H2SiO4-2] + [OH-] – [H+] CB (meq/L) = [H3SiO4-] + 2 • [H2SiO4-2] + [OH-] – [H+] Acidity can be determined empirically by titrating a solution with a strong base. Alkalinity can be determined empirically by titrating a solution with a strong acid. Acidity = 5 For a solution that begins with a strong acid or strong base the titration curve has an inflection at pH = 7. Around this inflection the curve is nearly a straight line and the addition of relatively little base causes a large change in pH. The amount of base required to reach this inflection is the acidity. For a mixture of a weak acid and a strong acid there are several inflection points around which the addition of relatively small amount of base causes a large change in pH. The amount of base required to reach each of these inflection points is defined as a different acidity – total acidity is the total amount of base required to reach the last inflection point. Note that alkalinity is the mirror image of acidity. Buffering = ability of a solution to resist changes in pH when H+ or OH- are added. The buffering capacity or index (B) is defined as B = dCB/dpH or B = dCA/dpH. The larger these numbers are the more OH- or H+ is required to create the same change in pH. Relatively small amount of H+ or OHrequired to change pH B is small. Relatively large amount of H+ or OHrequired to change pH B is large. Note that B is the slope of curve on an acidity or alkalinity titration curve To derive B theoretically (book page 80 – 87): 1. Write an equation for CB or CA 2. Differentiate these equations with respect to [H+] to obtain dCB/d[H+] or dCA/d[H+] 3. Use the fact that d[H+] = 2.3 • [H+] • dpH (derived from basic calculus) to convert these to dCB/dpH or dCA/dpH For a system containing a weak diprotic acid: B = 2.3 • ( Ka1• CA• [H+] + Ka2• CA• [H+] + Kw +] + [H (Ka1 + [H+])^2 (Ka2 + [H+])^2 [H+] ) Hint on problem set B = 2.3 • ( Ka1• CA• [H+] + Ka2• CA• [H+] + Kw +] + [H (Ka1 + [H+])^2 (Ka2 + [H+])^2 [H+] ) For problem 39 you will need to apply this equation to a solution containing H4SiO4. To do this you will need a value for CA. We derived an expression for CA in this system earlier in the class: CA(meq/L) = 2 • [H4SiO4] + [H3SiO4-] + [H+] – [OH-] But to use this you need values for [H4SiO4] and [H3SiO3-] Life will be easier if you use the facts that at pH < 9.83 [H4SiO4] >> [H3SiO4-] and you can assume that all the dissolved silicic acid is H4SiO4. At a pH of 9.83 [H4SiO4] = [H3SiO4-] and at pH > 9.83 [H3SiO4-] >> [H4SiO4] and you can assume that all dissolved silicic acid is H3SiO4-. 3 pts extra credit for people who can show why this is true. Buffering capacity of natural systems: The most effective common natural buffer is the carbonic acid – calcite buffer. Clay minerals have a significant theoretical ability to buffer acidic solutions through reactions like: 2KAl3Si3O10(OH)2 + 2H+ + 3 H ↔ 3 Al2Si2O5(OH)4 + K+ + H4SiO4 kaolinite muscovite aqueous But the kinetics of these reactions are relatively sluggish. Weathering reactions involving quartz, feldspars and ferromagnesium silicates (unweathered igneous rock) are relatively ineffective as buffers.