DENSITY
advertisement

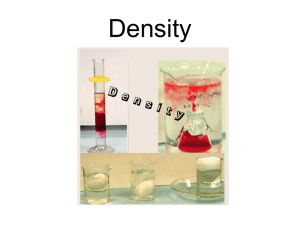
DENSITY 1 DENSITY Pb Pb Both of the above blocks consist of pure lead (Pb) 2 Pb Pb WHICH BLOCK HAS THE GREATER DENSITY? IF YOU ANSWERED, “THEY HAVE THE SAME DENSITY.” YOU ARE CORRECT!!! WHY? 3 WHY? 1. Both of the above blocks consist of pure lead (Pb) 2. Density is a ratio of Mass to Volume (D=M/V). Let’s do some calculations to explore this. 4 Let’s take measurements of each block. Small block: mass: 272.16 g Large Block: mass: 1360.8 g linear measurements: linear measurements: 2.00cm x 3.00cm x 4.00cm 4.00cm x 5.00cm x 6.00cm 5 Let’s calculate the volume of each block. Small block: mass: 272.16 g Large Block: mass: 1360.8 g Volume Calculations: Volume Calculationss: 2.00cm x 3.00cm x 4.00cm = 24.0 cm3 4.00cm x 5.00cm x 6.00cm = 120cm3 6 Now let’s calculate the density of each. Small block: mass: 272.16 g Volume: 24.0 cm3 Large Block: mass: 1360.8 g Volume: 120cm3 DENSITY=M/V DENSITY=M/V 272.16 g = 11.3 g 24.0cm3 cm3 1360.8g = 11.3g 120cm3 cm3 7 Small block: mass: 272.16 g Volume: 24 cm3 272.16g = 11.3 g =DENSITY 24.0cm3 cm3 Large Block: mass: 1360.8 g Volume: 120cm3 1360.8g = 11.3g = DENSITY 120cm3 cm3 Note: the mass to volume ratios (DENSITIES) are the same. 8 Density as a Conversion Factor Note: The units of density are in a ratio form, so density can be used as a conversion factor to change mass to volume or volume to mass. Let’s explore this. 9 Problem: Determine the mass of a 1.00 L sample of Pb. (The density of Pb is 11.3 g/cm3, 1 L = 1000mL =1000 cm3 Solution: 1. What are the units of what we are looking for? __________________ = g (mass units = grams) 2. What do we have? 1.00 L______________ = g 3. Now convert L into g using density 1.00 L 1000 mL 11.3 g 1L mL = 11300 g 10 Problem: Determine the volume of a 1.00 lb (454 g) sample of Pb. (The density of Pb is 11.3 g/cm3) Solution: 1. What are the units of what we are looking for? __________________ = cm3 (volume units) 2. What do we have? 1.00 lb______________ = cm3 3. Now convert lb into cm3 using density 1.00 lb 454 g 1.00 lb cm3 11.3 g = 40.17 cm3 40.2 cm3 11 Let’s compare the two solutions. 1.00 L 1000 mL 11.3 g 1L mL 1.00 lb 454 g 1.00 lb cm3 11.3 g = 11300 g = 40.17 cm3 Compare how density was used in the two solutions. 12 Now work with someone next to you to solve this problem. Problem: A rock having a mass of 136.17 g was placed in a graduated cylinder containing water with an initial reading of 23.0 mL. The water level rose to a reading of 48.5 mL. Calculate the density of the rock. Volume = 48.5 - 23.0 =25.5 mL Density = 136.17 g = 5.34 g = 5.34 g 25.5 mL mL cm3 13 Cooking oil has a density of approximately 0.952 g/mL. a. Determine the mass of 550 mL of cooking oil. 550 mL 0 .9 5 2 g mL = 523.6 g 520 g b. Determine the volume of 870 g of cooking oil. 870 g mL 0 .9 5 2 g = 913.9 910 mL mL 14 Densities of Some Common Materials Substance water (25oC) ice (0oC) Gold Lead sand Density g/cm3 1.00 0.917 19.31 11.3 ?? Does density change with temperature? 15