Density
advertisement

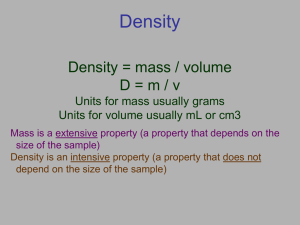
Density Density • Density is how compact matter is. • More mass shoved into a certain amount of space makes something more dense. Density Considers Two Things • How much mass is present. • How much volume does it take up. • Make sure to consider both these factors when discussing density. A A 1.What does Figure A illustrate about density? A A 2. What does Figure B illustrate about density? A A A A 3. If the cube of aluminum in figure B were cut into four equal parts. How would the density of one part compare to the whole cube? A A 4. Metals expand when heated. If the gold cube in part B is heated, what will happen to its density? Does it Float? • If something floats or rises it is less dense than the material it is floating/rising in. • Objects of greater density will sink. Density • Density is the mass per unit volume. – How much mass there is in a certain amount of space. Density • Density is the mass per unit volume. • Density = Mass ÷ Volume Density • Density is the mass per unit volume. • D = Mass ÷ Volume ÷ Solving For Variables Common Volume Units Mass = 2680g Volume = 1450cm3 Illegal ivory is often detected on the basis of density. What is the density of the elephant tusk? Mass = 2680g Volume = 1450cm3 D=M÷V = 2680g ÷ 1450cm3 = 1.8482g/cm3 = 1.85g/cm3 Illegal ivory is often detected on the basis of density. What is the density of the elephant tusk? Calcium chloride is used as a deicer on roads in winter. Its density is 2.50g/cm3. What mass of calcium chloride will the truck hold if its volume is 4560L? Calcium chloride is used as a deicer on roads in winter. Its density is 2.50g/cm3. What mass of calcium chloride will the truck hold if its volume is 4560L? 4560L = 4,560,000cm3 Calcium chloride is used as a deicer on roads in winter. Its density is 2.50g/cm3. What mass of calcium chloride will the truck hold if its volume is 4560L? 4560L = 4,560,000cm3 M=DxV = 2.50g/cm3 x 4,560,000cm3 = 11,400,000g = 11,400,000g The density of methanol is 0.788g/cm3. What is the minimum volume of a tank that can hold 795kg of methanol. The density of methanol is 0.788g/cm3. What is the minimum volume of a tank that can hold 795kg of methanol. 795kg = 795,000g The density of methanol is 0.788g/cm3. What is the minimum volume of a tank that can hold 795kg of methanol. 795kg = 795,000g V=M÷D = 795,000g ÷ 0.788g/cm3 = 1,008,883cm3 = 1,010,000cm3 How many liters is this? The density of methanol is 0.788g/cm3. What is the minimum volume of a tank that can hold 795kg of methanol. 1,010,000cm3 ÷ 1000 cm3/L = 1010L Homework • Worksheet: Chapter 2-3