Tammy`s slides - ISPJE Webinar
advertisement

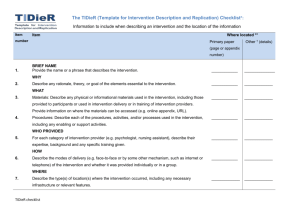
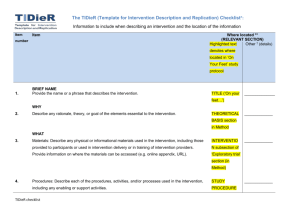
“But WHAT did they actually do?” Poor reporting of interventions: a remediable barrier to research translation Associate Professor Tammy Hoffmann @Tammy_Hoffmann thoffmann@bond.edu.au Waste in Research >85% of research is wasted due to: Chalmers, I. & Glasziou, P., 2009. Avoidable waste in the production and reporting of research evidence. Lancet, 374(9683), pp.86–9. Reporting guidelines • Although ultimate responsibility lies with researchers, editors “should take all reasonable steps to ensure the quality of the material they publish”. • Guidelines for reporting health research are important tools to facilitate this. Reporting Guidelines • Specify a minimum set of items needed for a complete and clear account of study methods and funding. • Adherence to guidelines improves the accuracy and transparency of publications. • Some journals refer authors to guidelines in ‘Instructions to Authors’ – has much less impact on reporting quality than requiring authors to adhere to them EQUATOR Network www.equator-network.org/ A guide for promoting the use of reporting guidelines in your journal: http://www.equator-network.org/wpcontent/uploads/2012/12/Reporting-guidelines-in-journals-August2013.pdf Most frequently used guidelines • CONSORT – for reporting of randomised controlled trials (RCTs) - currently >23 000 trials in PEDro • PRISMA – for systematic reviews and meta-analyses • STARD – for diagnostic accuracy studies • STROBE – for observational studies in epidemiology Without a complete description of an intervention… • other researchers cannot replicate or build on research findings • for effective interventions, clinicians, patients, and other decision makers are left unclear about how to reliably implement the intervention Do journals provide sufficient instructions to authors? • Audit of ‘Instructions to Authors’ for 106 journals • 58% mentioned the CONSORT statement + 6% also mentioned the CONSORT extension statements • Only 15 (14%) specifically mentioned the reporting of interventions Of these, nearly all provided non-specific instructions e.g. “Describe study procedures, including any interventions…” Hoffmann, T.C., English, T. & Glasziou Paul, P., 2014. Reporting of interventions in randomised controlled trials: an audit of journal Instructions to Authors. Trials, 15(20). How big is the problem? % of interventions rated as adequately described 100 90 80 After author contact Initially 70 60 50 40 30 20 10 0 Setting Recipient Provider Procedure Materials Intensity Schedule Individual checklist items and overall rating of completeness of the intervention description Hoffmann, T., Erueti, C., & Glasziou, P. (2013). BMJ. 347:f3755 Overall Aim of TIDieR To improve the completeness of reporting, and ultimately the replicability, of interventions emphasis is on trials, but the guidance is intended to apply across all evaluative study designs http://www.bmj.com/content/348/bmj.g1687.long TIDieR development process • Literature review Potential checklist items were generated from: – Existing checklists – Forward and backward citation searching of each published checklist – Research analysing the quality of intervention reporting in trials and related literature • Two round modified Delphi consensus survey • Face-to-face consensus meeting A recipe CONSORT + TIDieR • An extension of the CONSORT 2010 Statement: SPIRIT + TIDieR • An extension of: Online supplementary materials Published PROTOCOL WEBSITE Smartphone APP VIDEO of the procedure PHOTOS of materials Limits? Who should use TIDieR? Authors • • • • of trials of protocols of other evaluative study designs of systematic reviews Reviewers Editors and Journals - endorse TIDieR - modify their ‘Instructions to Authors’ and require its use (by authors AND reviewers) BMJ 2014;348:g1687 www.equator-network.org/reporting-guidelines/tidier/ Checklist available in PDF and Word template