Lecture 7
advertisement

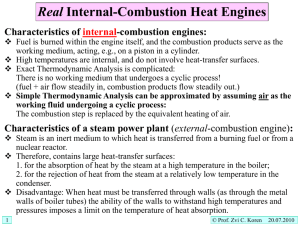
Paperwork • • • • • • • • Assignments fo 18&19 due next week Today 20.4-20.5 Problems for 18&19 Will finish chapter 20 Monday (Guest) Tuesday – Lab3 & Quiz3 Wed – Problems for Ch. 20 Fri – Ch.21 (Guest) Exam Following Friday Engines = Cyclic Processes • • • • • • • Initial State = Final State Q = DU + W What does this imply about DU? DU = UF – U0 = U – U = 0 Engine returns to starting state (p,V,T) But Heat can enter system (Fuel, Energy In) Work is Done (Energy Out) – Q=0+W Engine Some Energy tapped to do work. Some Lost. Not Path Independent means non-conservative! Heat not a liquid, but think H20 Tower QH Work QC Perpetual Motion? QH Work QC Why must some heat (energy) be lost to cold side? WaterWheel • Converts Water Flow (H20 KE) to Usable Energy (Wheel KE) WaterWheel – on a H20 Tower Tower Reservoir - Higher Graviational PE Not all H20 Energy Used Some flows to ground Could convert all? Ground - Lower Graviational PE WaterWheel – on a H20 Tower Tower Reservoir - Higher Graviational PE Stop flow down just means higher “ground” H20 KE less, also stops wheel from turning Same Problem with heat Need a “sink” to direct flow Perpetual Motion? QH Work QC Why must some heat (energy) be lost to cold side? No cold sink, no heat flow! [Warning heat is NOT a liquid] What Happens if I Reverse W? QH Work QC Work Entering System instead of Work being done by system. dQ = dU + W (Here, W <1) What Happens if I Reverse W? QH Work QC Work Entering System instead of Work being done by system. dQ = dU + W (Here, W <1) Can Make dQ negative (Flow backwards) Makes cold side colder? At least it removes heat energy QC is heat taken out of cold side (what you want done) W is work it takes to do this QH is heat that goes into hot side (QH>QC) Fridge exhaust - RT, Hotter or Colder? QH Work |QH|+|W|=|QC| QC Refrigerator Fridges have K, like efficiency: K QC W QC QH QC QH Work QC Efficiency • If 100% efficient – Perpetual Motion (patents everwhere…) • Engines – Not all Heat converted to work • Fridge – Need some work to force heat to flow from cold object to hot object • Statements = 2nd law of thermodynamics – No Free Lunch? ?’s Choose 1 • If you increase the temperature of an ideal gas? – – – – its volume must increase its pressure must increase the speed of its molecules must increase the average distance a molecule travels between collisions must increase – the average time between collisions of its molecules must increase. • Answer – speed. Implications of each? Choose 1? • Which of the following is FALSE? – A dumbbell molecule like O2 is considered to have eight degrees of freedom. – The average kinetic energy of a molecule moving in three dimensions is always 3kT/2. – Rotational motion, as well as translational motion, can contribute to the heat capacities of gases. – The average kinetic energy associated with each degree of freedom of a molecule is 1/2kT. – Vibrational motion, as well as translational motion, can contribute to the heat capacities of gases. • 5 not 8. Remainder? Choose 1 • Q = DU + W. Which of the following statements is FALSE? – W is the work done by the system, and not on the system. – Q can be positive or negative. – This is called the law of conservation of energy. – This is called the First Law of Thermodynamics. – It follows that since Q and W are path dependent, then DU must also be path dependent. • Last One – Internal Energy depends only on Temp for ideal gas (+ KE, PE’s, etc… for others) Choose Any • Which of the following are true statements about an ideal gas? (What is isochoric?) – During an isochoric process, the change in the internal energy of the gas is exactly equal to the amount of heat that goes into (or out of) the gas. – During an isothermal process, no heat enters or leaves the gas. – During an isochoric process, no work is done on or by the gas. – During an isothermal process, the temperature of the gas does not change. – During an adiabatic process, the temperature of the gas does not change. • Only 1st one. What about 3rd one? Choose any? • Which of the following is an accurate statement? – A typical gasoline engine has an efficiency of about 2%. – An important distinction between the Diesel cycle and the Otto cycle is that for the Diesel cycle high efficiencies may be obtained with low compression ratios. – An important distinction between the Diesel cycle and the Otto cycle is that there is no fuel in the cylinder at the beginning of the compression stroke and no spark plug is used. – Because a Diesel engine requires no fuel ignition system, Diesel engines tend to be lighter and easier to start than a comparable gasoline engine. – The efficiency of the Otto cycle does not depend on the compression ratio. • Middle: Implications of others? Friday • Return Quiz 1 • Cover one problem each from 18,19,20 – Or at least 18&19
![M&M Lab Report Template [11/1/2013]](http://s3.studylib.net/store/data/007173364_1-88fa2a4b33d860a6d8d06b9423fde5c0-300x300.png)