7. Energy, Power and Climate Change
advertisement

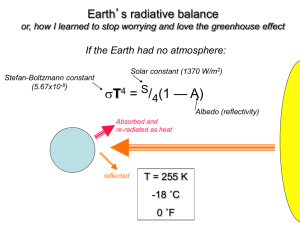
7. Energy, Power and Climate Change Chapter 7.2 - The greenhouse effect and global warming The black-body law • All object that are kept at some absolute temperature T (kelvin scale) radiate energy in the form of electromagnetic waves. • Depending on the temperature of the object, it will radiate different types of energy. • Some objects only emit IR radiation, like people but others can emit visible radiation, like a hot piece of iron. Stefan-Boltzmann Law • The power radiated by a body is governed by the StefanBoltzmann Law. The amount of energy per second radiated by a body depends on its surface area A, absolute temperature T and the properties of the surface P eAT 4 where = 5.67x10-8 W m-2 K-4 (Stefan-Boltzmann constant) e is the emissivity of the surface. It has no dimensions (units) and varies between 0 and 1). For a perfect emitter (black body) e =1. Black-body • A black body is an object that absorbs all electromagnetic radiation that falls on it. No electromagnetic radiation passes through it and none is reflected. Because no light (visible electromagnetic radiation) is reflected or transmitted, the object appears black when it is cold. • If the black body is hot, these properties make it an ideal source of thermal radiation. • A black body is thus an object that is a ‘perfect’ emitter of radiation. • A real body can be a good approximation to a black body if its surface is black and dull. Black-body • But a shiny and silver object is clearly the opposite to a black body as it reflects radiation rather that absorbing it. • Dark dull surfaces have e close to 1 they are almost perfect emitters and are a good approximation to a black body • Shiny silver surfaces have e close to 0 they cannot be considered black bodies. Black-body • A body of emissivity e that is kept at temperature T1 will radiate power at a rate Pout = e A T1 4 but will also absorb power at a rate Pin = e A T2 4 if the surroundings are kept at temperature T1. • Hence the net power lost by the body is Pnet = Pout - Pin = e A (T1 4 -T2 4) • At equilibrium, Pnet = 0, i.e., the body loses as much energy as it gains, and so the body’s temperature stays constant a equals that of the surroundings: T1 = T2. Black-body • The energy that is radiated by a body is electromagnetic radiation and is distributed over an infinite range of wavelengths. • However, most of the energy is radiated at a specific wavelength that is determined by the temperature of the body: The higher the temperature of the body, the shorter the wavelength for maximum emission. • For a body at room temperature (20ºC or 293K) the wavelength at which most radiation is emitted is an infrared wavelength. Black-body spectrum • Very hot objects will radiate at short wavelengths. Ex: the sun’s temperature is around 5500 K and its for maximum emission corresponds to green/yellow light. • Cold objects will radiate at long wavelengths. Ex: the human body radiates in the infrared region. Black-body radiation and Wien’s law • The relationship between the max and the object’s temperature is given by Wien’s law : max T 2.9010 m K 3 Power emission and emissivity For different surfaces at the same temperature (300K), the power emitted will be different and will depend of the surface’s emissivity e = 1.0 power emitted e = 0.8 e = 0.2 0.5 1.0 1.5 2.0 2.5 3.0 /x10-5 m Solar radiation • The sun may be considered to radiate as a perfect emitter, that is, as a black body. • The total power emitted by the Sun is P = 3.9 x 1026 W • The power received per unit area on earth is called intensity and given by: P I 4r 2 PIA • Substituting the numerical values gives I 3.91026 4 1.5 10 11 2 1400W/m2 • This is the intensity of the solar radiation at the top of earth’s atmosphere. It’s called solar constant and denoted by S. Albedo • The albedo, , of a body is defined as the ratio of the power of radiation reflected or scattered from the body to the total power incident on the body. t ot alscat t ered/reflect edpower t ot alincident power • The albedo is a dimensionless number. Snow (white shiny surfaces) has an albedo of 0.85. It reflects almost all radiation. Charcoal (dark dull surface) has an albedo on 0.04. It reflects very little radiation. • The earth as a whole has a global average albedo of about 0.3. The variations depend on: - the time of the year (many or few clouds) - latitude (amount of snow or ice) - whether one is over desert land (=0.3-0.4), forests (=0.1) or water (=0.1). Albedo • At any one moment in time, the earth offers a ‘target’ area R2, where R is the radius of the Earth. • As the target area is ¼ of the total surface area of the earth, the power radiation received per square metre of the Earth’s surface is S/4 = 350 W/m2. disc of • Since 30% is reflected (albedo), this means that the earth receives a net radiation intensity of 245 W/m2. solar radiation area R2 sphere of surface area 4R2 R radiation Energy balance • The earth has a constant average temperature and behaves as a black body. So the energy input to the earth must equal (balance) the energy output by the earth. • Taking account of the albedo, the power delivered to surface area A is P = (1 - ) IA • If the atmosphere is not taken into account, we can consider this energy diagram for earth (energy balance equation). incoming intensity = I reflected = I radiation back into space = (1 – ) I received by the earth = (1 -- ) I The Greenhouse effect • Any planet with an atmosphere experiences this effect. • Most radiation that reaches earth’s surface is in the visible region of the EMS, with small amounts of IR and UV. • About 30% is reflected back into space. • The rest of the radiation is absorbed by earth's atmosphere and surface. • Earth's average temperature is about 288K which means that it will radiate energy in the IR region. • Unlike Visible light, IR light does not pass through the atmosphere as it is strongly absorbed by various gases (greenhouse gases). The Greenhouse effect • This means that some of this radiation is received by the earth’s surface again, causing additional warming. • This is radiation that would be lost in space were it not for the greenhouse gases. • Without this greenhouse effect, the earth’s temperature would be 32K lower than what it is now (TEarth = 256K or -17°C). The Greenhouse effect incoming intensity radiated back into space greenhouse effect reflected absorbed IR radiation re-radiated back to earth earth’s surface received by earth and atmosphere The Greenhouse effect The greenhouse effect is the warming of the earth caused by infrared radiation, emitted by the earth’s surface, which is absorbed by various gases in the earth’s atmosphere and is then partly re-radiated towards the surface. The gases primarily responsible for this absorption (the greenhouse gases) are water vapour, carbon dioxide, methane and nitrous oxide. The Greenhouse effect The Greenhouse effect • The greenhouse effect is a natural consequence of the presence of the atmosphere. • However, there is an enhanced greenhouse effect which refers to additional warming due to increased quantities of the greenhouse gases in the atmosphere. • This increase is due to human activity. • Greenhouse gases can have natural or anthropogenic (man-made) origins. The Greenhouse effect • But there are also ways of reducing the concentrations of these gases: - CO2 is absorbed by plants during photosynthesis and dissolved in oceans; - CH4 (methane) is destroyed in the lower atmosphere by chemical reactions involving free hydroxyl radicals (HO·) - nitrous oxide is also destroyed in the lower atmosphere by photochemical reactions. • It must be noticed that most radiation coming from the sun is visible light which is not absorbed by the gases in the atmosphere. Therefore, the incident radiation passes through the atmosphere and arrives the earth’s surface. Sources of greenhouse gases Greenhouse gas H2O (g) Natural sources Anthropogenic causes Evaporation of water from oceans, rivers and lakes CO2 Forest fires, volcanic Burning fossil fuels in power plants eruptions, evaporation of and cars, burning forests water from oceans CH4 Wetlands, oceans, lakes and rivers Flooded rice fields, farm animals, termites, processing of coal, natural gas and oil, and burning biomass N 2O Forests, oceans, soils and grasslands Burning fossil fuels, manufacture of cement, fertilizers, deforestation (reduction of nitrogen fixation by plants) Mechanism of photon absorption • Electrons within atoms can absorb have quantized energies. • In the same way, molecules can absorb discrete amounts of energy related to their vibrational and rotational motion. • Just like electrons exit in energy levels in atoms, there are vibrational and rotational energy levels. • The difference, however, is that the energy difference between rotational/vibrational energy levels is the same as the energy of IR photons (atomic levels have difference in energies related to UV, Vis and IR Mechanism of photon absorption • This means that when IR photons travel through a greenhouse gas, they will be absorbed. • As the excited state is an unstable one, the radiation absorbed will be emitted again, but this time in all directions. • That is, the photons are not all emitted outwards into space. Some are emitted back towards the earth, thereby warming the earth’s surface. incoming intensity radiated back into space greenhouse effect reflected absorbed IR radiation re-radiated back to earth received by earth and atmosphere earth’s surface Mechanism of photon absorption • Consider two atoms forming a diatomic molecule. • The force between atoms may be loosely modelled as a massspring system. • Simple harmonic oscillations take place when the atoms are disturbed from their equilibrium positions. • The frequency of oscillations is given by: 1 f 2 k m where m is related to the mass of the 2 atoms m1 and m2, through: m1m2 m m1 m2 Mechanism of photon absorption • For CO2, k=1900 N/m and m=1.14x10-26 kg. So f = 6.5x1013 Hz. • This is the natural frequency of the molecule. • Photons travelling through this gas will be in resonance with the molecule if they have a frequency equal to the natural frequency. • A typical IR photon as E = 0.25eV and, therefore, f = 6.1x1013 Hz. • This means that the photon will be absorbed by the molecule Transmittance curves • Consider IR radiation passing through the atmosphere. • Some of this radiation will be absorbed so the intensity of the radiation after passing through the atmosphere will be reduced. • A transmittance curve shows how percentage of radiation transmitted by the gas varies with the wavelength. T/% 100 6 12 /m Transmittance curves • The black-body spectrum of the sun is not a perfect curve (doted line) as the gases present in the atmosphere absorb part of the radiation. H2O CO2 Transmittance curves Surface heat capacity • We define CS, the surface heat capacity of the body, as the energy required to increase the temperature of 1m2 of the surface by 1 K. • The concept is useful in the context of bodies radiating and absorbing energy, since it is the surface that is responsible for the energy lost or gained. • The units of CS are J m-2 K-1. • Thus, for a surface of surface heat capacity CS and area A, the amount of thermal energy needed to increase its temperature by T is given by: Q ACS T Surface heat capacity – example question Q4 Show that CS is related to c (specific heat capacity). Q ACS T and Q m cT ACS T mcT But m V Ah and hence: ACS T AhcT CS hc so: Global warming • The next figure shows the variation of the deviations of the earth’s average T from the expected long-term average since 1880. Global warming Global warming – concentration of CO2 over the years • CO2 concentration has doubled since the nineteenth century (pre-industrial times). • There seems to be a connection between global warming and greenhouse gases but some say that the data collected does not cover a large enough time span. Global warming – concentration of CO2 over the years • However, analysing samples from ice cores in Antarctica and Greenland it is possible to measure the concentration of different gases at the time of freezing. • The results show that there is a very close link between global warming and increased greenhouse gas concentrations. • Some of the Antarctic ice cores are extracted form a depth of about 3600m over the frozen lake Vostok. • They reveal a connection between temperature changes and changes in CO2 and CH4 concentrations. Global warming – concentration of CO2 over the years • The ice cores give a detailed account of global climate conditions over a time period spanning some 420 000 years. The figure below shows how T and [CO2] have varied over time. Global warming – concentration of CO2 over the years • The figure below shows that the earth was warmest whenever the levels of CO2 in the atmosphere increased. • Looking at the present concentration of CO2 it is certain that temperature will increase. • What is uncertain is the detailed effect of this temperature increase on the global climate – in magnitude and in time scale. Global warming Global warming Global warming • The majority of experts tend to agree that the enhanced greenhouse effect is behind global warming. • But some say that GW may be due to an increase solar power output as solar activity has a periodic variation. • However, the pattern of GW is not consistent with this variation. • Other theories include the increase of volcanic activity, variations of eccentricity of the earth’s orbit around the sun as well as ‘tilt’ of the orbit with respect to the sun. • These orbital phenomena occur over time scales ranging from 20 000 to 100 000 years which means that they are not so relevant when only the last 200 years are considered. Sea level • The level of water is always varying due to changes in atmospheric pressure, plate tectonic movements, wins, tides, flow of large rivers into the sea, changes in water salinity and others. • But the problem is when sea level changes because of climate changes. It is know that climate changes affect sea level through the fact that the temperature determines how much ice melts or how much water freezes. • During the last ice age (about 18 000 years ago), the sea level was about 100m lower than it is today. Changes in sea level affect the amount of water that can evaporate and the amount of thermal energy that can be exchanged with the atmosphere. In addition, changes in sea level affect ocean currents which are vital in transferring thermal energy from the warm tropics to colder regions The melting of ice • To melt a mass m of ice requires an amount of thermal energy Q=mLf. • When it comes to discuss sea level, we must distinguish between land ice (ice supported on land) and sea ice (ice floating in sea water). • When sea ice melts, there is no change in sea level. This is a consequence of Archimedes’ principle – the weight of the ice equals to the weight of the displaced water. So when ice melts, the resulting water will occupy the place of ice and no change will take place. • However, when land ice melts, the water will eventually flow to the sea and will increase the sea level. Estimating changes in sea level • Another important aspect of the melting of land ice is that it will expose the dark land underneath the ice. The dark land absorbs light very easily and will radiate IR radiation that will contribute to the increase of global temperature. • Sea level will increase because - more land ice will melt; - warmer water occupies a larger volume. • However, water expansion is anomalous. Water: - contracts in volume when it goes from 0ºC to 4ºC - expands as the temperature is increased further from 4ºC. • This means that the density of water is highest at T=4ºC and this fact has a great importance for life in lakes, rivers and oceans. • Given a volume V0 at a temperature 0, the volume after a temperature increase of will increase by V given by Estimating changes in sea level • Given a volume V0 at a temperature 0, the volume after a temperature increase of will increase by V given by V = V0 where is a coefficient known as coefficient of volume expansion • The coefficient of volume expansion is defined as the fractional change in volume per unit temperature change. • For water, the coefficient actually depends on temperature, and so a given volume of water will change by different amounts even for the same temperature changes depending on the initial temperature of water. Effects of global warming on climate • A higher average earth temperature implies a rising sea level. • One effect on climate of a rising sea level is the change in the albedo of the surface (more water as opposed to dry land). • The change in the albedo will not change significantly the temperature. • However, warmer water at higher temperature evaporates more easily and more water vapour will be released to the atmosphere. • This means: - cooling of the earth’s surface, - more cloud cover (and more reflected radiation) - more precipitation (rain may not fall in the region of interest) Effects of global warming on climate • Another effect of higher T is that the solubility of CO2 in the oceans decreases. This means more CO2 is left in the atmosphere. • Deforestation is a controversial issue. • Rain forests produce CH4 and thus contribute to increased concentrations of greenhouse gases • They absorb CO2 from the atmosphere during photosynthesis but return that CO2 when they die and decompose. Measures to reduce global warming • Using fuel-efficient cars and developing hybrid cars further; • Increasing the efficiency of coal-burning power plants; • Replacing coal-burning power plants with natural gas-fired plants; • Considering methods of capturing and storing the CO2 produced in power plants; • Increasing the amounts of power produced by wind and solar generators; • Considering nuclear power; • Being energy conscientious, with buildings, appliances, transportation, industrial processes and entertainment; • Stopping deforestation. The Kyoto protocol and the IPCC (intergovernmental panel on climate change) • An extremely important agreement towards cutting greenhouse gas emissions was reached in 1997, in Kyoto, Japan. • The industrial nations agreed to reduce their emissions of GG by 5.2% from 1990 levels between 2008 and 2012. • The protocol allowed mechanisms for developed nations to use projects aimed at reducing emissions in developing nations as part of their own reduction targets. • Endorsed by 160 countries, the protocol would become legally binding if at least 33 countries signed it. • The non-ratification by the USA and Australia has weakened the impact of the agreement. The Kyoto protocol Participation in the Kyoto Protocol Signed and ratified Signed, ratification pending Signed, ratification declined Non-signatory The Kyoto protocol and the IPCC (intergovernmental panel on climate change) • Unlike Kyoto protocol, which imposed mandatory limits for GG emissions, the Asia-Pacific Partnership on Clean Development and Climate asked for voluntary reductions of these emissions. • It was signed by the USA, Australia, India, China, Japan and South Korea in 2005. • It is an agreement in which the signatory nations agree to cooperate in reducing emissions. • It has been criticized as worthless because the reductions are voluntary but defended because it includes China and India, major GG producers, who are not bond by the Kyoto protocol. The Kyoto protocol and the IPCC (intergovernmental panel on climate change) • A major, comprehensive, detailed and scientifically impartial analysis of global climate has been undertaken by IPCC. • The IPCC was created by the World Meteorological Organization and the United Nations Environment Programme in 1988. • It does no research of its own but it collects information from scientific material to report on technical and socio-economic aspects of climate change. • Its 4 reports (1990, 1997, 2001 and 2007) have been instrumental in providing an accurate analysis of the global situation.