NOT
advertisement

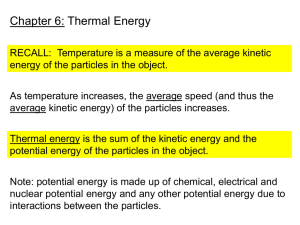
Heat & Thermodynamics Test Prep Game 1) What is the energy transferred to or from a unit of mass of a substance during a phase change called? a) Latent Heat b) Specific Heat Capacity c) Internal Energy d) Thermal Energy 1) What is the energy transferred to or from a unit of mass of a substance during a phase change called? a) Latent Heat b) Specific Heat Capacity c) Internal Energy d) Thermal Energy 2) A 0.075 kg object at 93 C is placed in 0.150 kg water with an initial temperature of 25 C. The final temperature of the object and water is 29 C. What is the specific heat capacity of the object (Cpw = 4186 J/kg C)? a) 260 J/kg C b) 520 J/kg C c) 129 J/kg C d) 1040 J/kg C 2) A 0.075 kg object at 93 C is placed in 0.150 kg water with an initial temperature of 25 C. The final temperature of the object and water is 29 C. What is the specific heat capacity of the object (Cpw = 4186 J/kg C)? a) 260 J/kg C b) 520 J/kg C c) 129 J/kg C d) 1040 J/kg C 3) How much work is done by 0.020 m3 of gas if it’s pressure increases by 2.0 x 105 Pa and the volume remains constant? a) -4000 J b) +4000 J c) 0 J d) 1 x 107 J 3) How much work is done by 0.020 m3 of gas if it’s pressure increases by 2.0 x 105 Pa and the volume remains constant? a) -4000 J b) +4000 J c) 0 J d) 1 x 107 J 4) A volume of cool air rapidly descends from the top of a mountain. The temperature increases as the volume decreases and the pressure increases. Which thermodynamic process is taking place? a) Adiabatic b) Isovolumetric c) Isobaric d) Isothermal 4) A volume of cool air rapidly descends from the top of a mountain. The temperature increases as the volume decreases and the pressure increases. Which thermodynamic process is taking place? a) Adiabatic b) Isovolumetric c) Isobaric d) Isothermal 5) Gas within a piston is compressed from 1.55 x 10-2 m3 to 9.5 x 10-3 m3. what quantity of work is done if the compression occurs at a constant pressure of 3.0 x 105 Pa? a) 1800 J b) 3600 J c) -3600 J d) -1800 J 5) Gas within a piston is compressed from 1.55 x 10-2 m3 to 9.5 x 10-3 m3. what quantity of work is done if the compression occurs at a constant pressure of 3.0 x 105 Pa? a) 1800 J b) 3600 J c) -3600 J d) -1800 J 6) A steam engine takes in 2750 J of energy as heat, gives up 1550 J of energy to the surroundings and does 850 J of work. What is the change in internal energy of the engine? a) 5150 J b) 3450 J c) 2050 J d) 350 J 6) A steam engine takes in 2750 J of energy as heat, gives up 1550 J of energy to the surroundings and does 850 J of work. What is the change in internal energy of the engine? a) 5150 J b) 3450 J c) 2050 J d) 350 J 7) Which of the following best describes a state of Thermal Equilibrium? a) Both systems have the same mass. b) Both systems have the same volume. c) Both systems have the same temperature. d) Both systems contain the same amount of internal energy. 7) Which of the following best describes a state of Thermal Equilibrium? a) Both systems have the same mass. b) Both systems have the same volume. c) Both systems have the same temperature. d) Both systems contain the same amount of internal energy. 8) Which of the following correctly describes Thermal Expansion? As the temperature increases, a) the volume of the substance increases b) the volume of the substance decreases c) the density of the substance increases d) the density of the substance decreases 8) Which of the following correctly describes Thermal Expansion? As the temperature increases, a) the volume of the substance increases b) the volume of the substance decreases c) the density of the substance increases d) the density of the substance decreases 9) What is 235 K when measured in degrees Celsius? a) 508 C b) 203 C c) -38 C d) -68 C 9) What is 235 K when measured in degrees Celsius? a) 508 C b) 203 C c) -38 C d) -68 C 10) Which temperature is widely used in science, and applied to non-scientific uses throughout most of the world? a) Rankine b) Celsius c) Fahrenheit d) Kelvin 10) Which temperature is widely used in science, and applied to non-scientific uses throughout most of the world? a) Rankine b) Celsius c) Fahrenheit d) Kelvin 11) Convert 36.7 C to degrees Fahrenheit. a) 309.7 F b) 123.7 F c) 52.4 F d) 98.1 F 11) Convert 36.7 C to degrees Fahrenheit. a) 309.7 F b) 123.7 F c) 52.4 F d) 98.1 F 12) What term is defined as the energy transferred between objects with different temperatures? a) Internal Energy b)Work c) Heat d)Thermal Equilibrium 12) What term is defined as the energy transferred between objects with different temperatures? a) Internal Energy b)Work c) Heat d)Thermal Equilibrium 13) What must be true for energy to be transferred between two bodies? The two bodies must: a) have different volumes b) be at different temperatures c) have different masses d) be in thermal equilibrium 13) What must be true for energy to be transferred between two bodies? The two bodies must: a) have different volumes b) be at different temperatures c) have different masses d) be in thermal equilibrium 14) If energy is transferred from an object with a temperature, T1, to an object with a temperature, T2, what must be true? a) T1 < T2 b)T1 = T2 c)T1 > T2 d)More information is needed 14) If energy is transferred from an object with a temperature, T1, to an object with a temperature, T2, what must be true? a) T1 < T2 b)T1 = T2 c)T1 > T2 d)More information is needed 15) What is the process by which energy is transferred by the motion of cold and hot material? a) Conduction b) Insulation c) Convection d) Radiation 15) What is the process by which energy is transferred by the motion of cold and hot material? a) Conduction b) Insulation c) Convection d) Radiation 16) Which of the following is NOT a good thermal insulator? a) Ceramic b) Iron c) Fiberglass d) Cork 16) Which of the following is NOT a good thermal insulator? a) Ceramic d b) Iron c) Fiberglass d) Cork 17) A 0.45 kg stone is dropped from a cliff. When the stone strikes the ground, the internal energy of the stone and ground increases by 1770 J. If the stone was initially at rest, how high was the cliff? a) 206 m b) 177 m c) 401 m d) 802 m 17) A 0.45 kg stone is dropped from a cliff. When the stone strikes the ground, the internal energy of the stone and ground increases by 1770 J. If the stone was initially at rest, how high was the cliff? a) 206 m b) 177 m d c) 401 m d) 802 m 18) What is the quantity of energy needed to raise the temperature of a unit mass of a substance by 1 C called? a) Latent Heat b) Specific Heat Capacity c) Internal Energy d) Thermal Energy 18) What is the quantity of energy needed to raise the temperature of a unit mass of a substance by 1 C called? a) Latent Heat d b) Specific Heat Capacity c) Internal Energy d) Thermal Energy 19) Which property of a substance is NOT needed to determine the amount of energy transferred as heat to or from a substance? a) Temperature b) Specific Heat Capacity c) Volume d) Mass 19) Which property of a substance is NOT needed to determine the amount of energy transferred as heat to or from a substance? a) Temperature b) Specific Heat Capacity d c) Volume d) Mass 20) The entropy of a system can increase, decrease or remain constant, but the total entropy of the universe is always _______. a) Decreasing b) Increasing c) Negative d) Zero 20) The entropy of a system can increase, decrease or remain constant, but the total entropy of the universe is always _______. a) Decreasing d b) Increasing c) Negative d) Zero 21) A 0.25 kg metal bolt gives up 3.6 x 103 J of energy as heat to the surrounding water. The specific heat capacity of the bolt is 360 J/kg C. What is the change in the bolt’s temperature? a) 0.40 C b) 2.5 C c) 4.0 C d) 40.0 C 21) A 0.25 kg metal bolt gives up 3.6 x 103 J of energy as heat to the surrounding water. The specific heat capacity of the bolt is 360 J/kg C. What is the change in the bolt’s temperature? a) 0.40 C b) 2.5 C c) 4.0 C d d) 40.0 C 22) An engine takes in 7.6 x 105 J of energy as heat and gives up 5.7 x 105 J of heat to the surroundings. What is the efficiency of the engine? a) 33% b) 25% c) 50% d) 46% 22) An engine takes in 7.6 x 105 J of energy as heat and gives up 5.7 x 105 J of heat to the surroundings. What is the efficiency of the engine? a) 33% d b) 25% c) 50% d) 46% 23) What are the units for Specific Heat? a) J/kgF b) Jm/kgC c) J/kgC d) N/kgsecC 23) What are the units for Specific Heat? a) J/kgF b) Jm/kgC d c) J/kgC d) N/kgsecC 24) What is the First Law of Thermal Dynamics? a) U = Q + W b) U = Q – W c) U = Q x W d) U = Q/W 24) What is the First Law of Thermal Dynamics? a) U = Q + W b) U =d Q – W c) U = Q x W d) U = Q/W 25) A wok is used to heat 100.0 g of olive oil from 20.0 C to 190 C. The specific heat of the oil is 2000 J/kg C. How much head was added to the oil? a) 3,400 J b) 17,000 J c) 68,000 J d) 34,000 J 25) A wok is used to heat 100.0 g of olive oil from 20.0 C to 190 C. The specific heat of the oil is 2000 J/kg C. How much head was added to the oil? a) 3,400 J b) 17,000 J c) 68,000 J d d) 34,000 J 26) An engine uses 5 x 106 J of heat. If the engine is 40% efficient, how much work can it do? a) 2 x 106 J b) 1.25 x 107 J c) 3 x 106 J d) 8.3 x 106 J 26) An engine uses 5 x 106 J of heat. If the engine is 40% efficient, how much work can it do? a) 2 xd 106 J b) 1.25 x 107 J c) 3 x 106 J d) 8.3 x 106 J 27) The form of heat transfer that takes place in a house heated by forced air is primarily: a) Radiation b) Work c) Conduction d) Convection 27) The form of heat transfer that takes place in a house heated by forced air is primarily: a) Radiation b) Work c) Conduction d d) Convection 28) Entropy states that natural processes tend to move towards greater: a) Order b) Disorder c) Total Energy d) Thermal Energy 28) Entropy states that natural processes tend to move towards greater: a) Order d b) Disorder c) Total Energy d) Thermal Energy 29) ________________ is a measure of the average kinetic energy of the particles in a substance. a)Temperature b) Internal Energy c) Thermal Equilibrium d) Latent Heat 29) ________________ is a measure of the average kinetic energy of the particles in a substance. d a)Temperature b) Internal Energy c) Thermal Equilibrium d) Latent Heat 30) Work done on or by a gas is dependent upon __________ and ____________ change. a)Heat, Volume b) Volume, Heat c) Pressure, Volume d) Internal Energy, Pressure 30) Work done on or by a gas is dependent upon __________ and ____________ change. a)Heat, Volume b) Volume, Heat d Volume c) Pressure, d) Internal Energy, Pressure