lou-armentano
advertisement

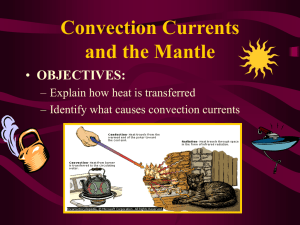
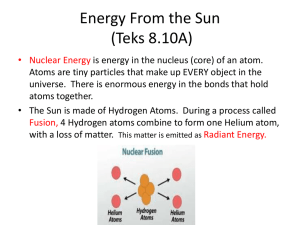
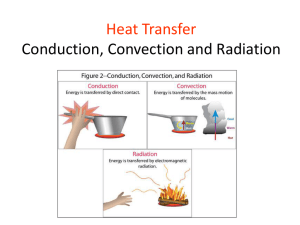
Heat transfer, heat production and temperature regulation in animals Physics Spring 2012 Lou Armentano learment@wisc.edu 263-3490 Why does temperature matter? • chemical reactions of metabolism are slower at low temperatures – rate approximately doubles per 10o C rise • proteins (enzymes, structural proteins), DNA and RNA denature at high temperatures • lipids in membrane require temperature to maintain fluidity for function – fatty acid profile can differ depending on species habitat temperature All animals produce heat • First law of thermodynamics, about the conservation of energy: • Second law of thermodynamics, about entropy • so they all must lose heat to environment or will cook themselves to death • if they lose heat faster than they generate it, body temperature falls • and vice-versa Heat transfer • Conduction • Convection – forced or natural • Radiation • Evaporation – heat loss only • in all cases surface area is important Conduction • heat transfer between non-moving matter • within on matter or between two touching matters • solid to solid, solid to unmoving fluid, unmoving fluid to unmoving fluid • fluid is liquid (like water) or gas (like air) Conduction and heat of fusion Convection • movement between a body and a moving fluid • fluid movement can be generated by heat – natural or passive convection • fluid movement can be forced – water pump forcefully moves engine coolant through engine convecting heat from cylinders to coolant and carrying it to radiator where it convects out of coolant to solid radiator and convects off of radiator to air – cooling fan and car movement forcefully convect air through radiator – heart forces blood from body core to surface – fans cool (or heat) body by forced convection Conduction (+ some Convection) to liquid Evaporation when emerges from pond Forced convection Reducing convective loss with animal fibers Radiation • electro magnetic transfer of heat • can operate through a vacuum • long distance (sun) P = rate of heat transfer, A = area, T = temperature Radiation Avoiding heat gain Seeking heat gain – notice surface area exposure and shape change Evaporation • latent heat of vaporization of water – 540 kcal/kg water evaporated – 2260 joule/g water evaporated • can cool animal when environmental temperature exceeds body temperature • cannot cool animal if air is saturated with water (100% relative humidity at skin temperature) • THI (temperature humidity index) – “its not the heat its the humidity” – its both • air movement replaces more water saturated air with fresh drier air Evaporation Forced convection with evaporation Note flattened tongue to increase surface area Heat can transfer into animal Radiation Forced Convection (pumps active) Conduction (pumps off, people still) Conduction • heat loss between unmoving matter (solids or still fluids) • Q/time = conductance (k)/depth (L) * area * temperature difference • heat loss/area at fixed temp difference = k / L • L is ‘depth’ of solid • k (conductance) is proportional to density – Insulating materials (styrofoam, fiberglass blankets, down jackets) are all low density to resist heat – One reason why loosing heat to air requires convection American goldfinch In this species feathers change with season (bright yellow in summer mating season)– but piloerection allows instantaneous adaptation to hot and cold temperatures (and sitting vs. flying) Conduction • heat loss between unmoving matter (solids or still fluids) • Q/time = heat loss • heat loss/area = k / L * temperature difference • l is ‘depth’ of solid • k is proportional to density • creating an unmoving layer of low density air = smaller k • increasing L reduces rate of heat transfer • fluffy hair, fur or feathers do both! • Offsets increased delta T in winter Convection • Rate of heat transfer by convection = h*A*(Ts - Tb) Ts=surface temperature (at interface of solid and fluid) Tb = temperature somewhere far enough from the surface so its the average temperature of the mass of fluid (think air temperature) A is the contact area Camels Temperature can vary by 6 degrees C in camels deprived of water – slows heat gain by convection during day, increase loss at night Heat transfer depends on • temperature difference determines direction and rate • area • how does area relate to animal mass? Not all animals share same shape volume (or mass) vs. area sphere Volume = 4/3 r3 Surface = 4 r2 cylinder Volume = r2 x height Surface = 2 rh + 2 r2 cube Volume =L3 Surface = 6 * L2 square-cube law Area increases by square of “length” Volume increases by cube of “length” even if shape described by multiple ‘lengths’ (like radius and height of a cylinder) all “lengths” increase proportionally to maintain shape (cylinder gets bigger or smaller but not relatively skinner or fatter) same shaped cylinders (r = x*h) different “shaped” cylinders (have different “B”-see following slides) Power function and log-log transform Y = B * Xz log Y = log B + Z * log X 2/3 2/3 power rule: area = B * mass B varies (slightly) with shape log surface area 5 y = 0.6667x + 0.6845 4 y = 0.6667x + 0.7432 y = 0.6667x + 0.7782 3 sphere 2 cylinder, h=2r 1 cube 0 0 1 2 3 4 log volume (or mass at constant density) 5 6 Same basic shape – markedly different size Giant and miniature Schnauzers Size variation exists in nature too – think asses vs. horses, foxes vs. wolves but variation has been exaggerated by artificial selection in domestic species Relationship of heat production and mass in adult homeotherms log(10)BMR (kcal/d) = 1.83 + .756*log BW in kg BMR = 67.6 * BW.756 BMR = 69 * BW .75 Kleiber, Fire of life Not just homeotherms Jack rabbits ears – blood flow increases (internal forced convection) when radiation/convection from ears allows cooling Animal is really a collection of shapes piloerection (physiological response) and flocking (behavioral response) both decrease convection note fluffier down type feathers in chick Shape and surface area important for functions other than heat • chicks don’t swim or fly so drag not important – have a relatively fixed solution in downy feathers • adult birds must be able to keep warm when still but resist drag when swimming or flying – piloerection allows a flexible solution rete mirabile – counter current for heat exchange (forced convection + conduction) Penquin feet: Counter current circulation keeps feet just above 0 C – venous blood extracts heat from arteries going to feet (feet conduct heat to ice) rete mirabile and sinus evaporation Brain (site of thermoregulation) remains cooler than body during heat stress Exercise 1 • What is heat loss by 70 kg homeotherm? • If reduce heat loss by 10% what is ‘excess’ heat? • how much will this raise body temperature in a day? • how much water would you need to evaporate to restore balance? • how much ice water would you need to drink to restore balance Solution • Daily heat produced = heat lost = 69*70.75 = 1670 kcal/d • 10% reduction in loss = 167 extra kcal • this heats a mass of 70 kg water by 167/70 = 2.4oC • 167/539 = .31 kg or L of water evaporated • 167/37 = 4.5 L water heated from 0 to 37oC