Glowacki-AT207
advertisement

Atmospheric chemistry
Lecture 1:
• Introduction & Overview
• Structure of the atmosphere
• Atmospheric Transport
Dr. David Glowacki
University of Bristol,UK
david.r.glowacki@bristol.ac.uk
Our goals in these lectures…
• Atmospheric Chemistry is
fascinating because it is spans a
range of fascinating subjects
• In one week, I hope to:
– give you an overview of
atmospheric chemistry
– teach you some of the key
principles
– provide you sufficient
background to understand
the details of two key arctic
atmospheric phenomena:
(1) arctic haze
(2) Polar ozone holes
Useful Reading Materials…
• Daniel Jacob, Introduction to Atmospheric Chemistry,
1999, available on the web at
http://acmg.seas.harvard.edu/publications/jacobbook/index.html
• Seinfeld & Pandis, Atmospheric Chemistry And Physics:
From Air Pollution to Climate Change
• Progress and Problems in Atmospheric Chemistry,
edited by John R. Barker
• G Marston, “Atmospheric Chemistry”, Annu. Rep. Prog.
Chem., Sect. C, 1999, 95, p 235-276
• G.E. Shaw, “The Arctic Haze Phenomenon”, Bull. Am.
Met. Soc., 1995, 76(12), p 2403-2413
• P.S. Monks, “Gas phase radical chemistry in the
troposphere”, Chem Soc. Reviews, 2005, p 2–21
Where I live…
What I do…
• I work on the frontier where chemistry
meets theoretical physics
• I use the mathematical tools of quantum &
classical mechanics to understand what
molecules do
• Most of my research involves massively
parallel computers
• A lot of what I do concerns how to make
more accurate approximations to solving
the full quantum mechanical equations
• Lots of applications:
• Atmospheric chemistry
• Combustion
• Materials Science
• Biochemistry
My background…
• During my PhD, I did atmospheric
chemistry experiments:
• Instrument design
• Laser spectroscopy
• Optics
• Chemical kinetics
http://www.chem.leeds.ac.uk/HIRAC/
Before my PhD…
• MA in religion and Cultural Theory at the University of Manchester:
• Undergraduate degree at the University of Pennsylvania in Philadelphia:
• Major in Chemistry with lots of work in math, physics, and Humanities
subjects
• That’s where I met Mark Hermanson
• We worked together to teach an environmental chemistry class
• Originally from Milwaukee, WI
Our Plan for Today’s Lecture
– The general structure of the atmosphere
Vertical Mixing in the Atmosphere
– Variation of Pressure with altitude
– Variation of Temperature with altitude
Horizontal Mixing in the Atmosphere
– Coriolis Forces
– Hadley Circulation
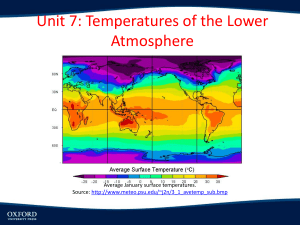
Atmospheric Temperature and pressure
variations
z
Heating by
exothermic
photochemical
reactions
Convective heating from
surface. Absorption of
IR (and some VIS-UV)
radiation from the sun
Vertical Mixing Processes
Variation of pressure with Altitude: The
hydrostatic equation
[P(z)-P(z+dz)]A
P(z+dz)
z
dz
P(z)
-ρgAdz
• Consider a column of air at altitude z
• A cross section of the air has width dz,
• It has two opposing forces:
• Upward direction: [P(z)-P(z+dz)]A
• Downward direction: -ρgAdz
• If the air parcel is in equilibrium, then:
[P(z)-P(z+dz)]A = -ρgAdz
[P(z)-P(z+dz)] = -ρgdz
Rewriting gives the hydrostatic equation:
p
dz
g (z )
Combining the hydrostatic equation with the
Ideal gas Law: the Barometric equation
[P(z)-P(z+dz)]A
P(z+dz)
z
dz
P(z)
• Ideal gas law tells us that
PV=nRT
• The Density of a gas, ρ, may thus
be written as:
= m(n/V) = m(P/RT)
where m is the molecular weight of
the gas
-ρgAdz
• Plugging this expression for density into the
hydrostatic formula gives:
• Rearranging and integrating we
obtain the Barometric equation:
p( z)
p0
p( z)
dz
exp( z / H s ) where
M air gp ( z)
RT (z )
Hs
RT
M air g
Properties of the barometric equation
p( z)
p0
exp( z / H s ) where
Hs
RT
M air g
• Hs is termed the scale height
• It is the
altitude over which the pressure falls by a factor of 1/e (0.37)
{hint: you can see this by setting z = Hs}
• The Barometric equation written above:
- Assumes T is constant (remember that T actually depends on
z!)
- May be compared with a Boltzmann distribution
- Has an average Mair = 28.8 g mol-1
- Hs = 6 km for T = 210 K; and ~8,5 km for T = 290 K.
- Species with a smaller molecular mass would have a larger
scale height; however, because of turbulent mixing, this
separation is not important in the troposphere and stratosphere
A simple application of the barometric
equation: sea breeze
p(z)
exp( z / H )
p0
ln[ p( z)] ln[ p(0)] Z
Mg
RT
• Fluids flow from regions of
high density (pressure) to
low density (pressure)
Mass conservation
Variation of Temperature with altitude: the
dry adabiatic lapse rate
Δ work
Δ Heat
(pressure – volume)
Δ System
Energy
st
1 law of Thermodyamics
(Conservation of Energy)
dU dq dw dq pdV
Δ enthalpy
The air parcel doesn’t
exchange heat with the
surroundings (adiabatic
process)
Tells how much energy we
have to put into the system to
change its temperature
dH dU pdV Vdp
dq 0
dH Vdp
dH mC p dT
mC p dT Vdp V ( gdZ )
letting
m
V
Dry Adiabatic dT g
d
Lapse Rate
dz
Cp
From the hydrostatic
equation
(dp/dz=-ρg)
The adiabatic lapse rate
d
•
•
•
•
•
dT
dz
As air parcels rise, they expand and cool
On earth, g and Cp combine
to give
d ~ 9.8 K
-1
km
The actual atmospheric temperature
gradient, is defined as:
= -(dT/dz)atm
The adiabatic lapse rate may be less
than or equal to
This affects vertical mixing, giving rise to
either stable or unstable conditions
g
Cp
Adiabatic lapse
Stable Atmosphere
• If d > the atmosphere is stable
& little mixing occurs
• As a rising air packet A expands, it
cools faster than the surroundings
• At the same pressure, TA(z+dz) <
TATM(z+dz), making A cooler and
denser ( P/T) than its
surroundings, slowing its rise
d
dT
dz
g
Cp
Convective Atmosphere
• If d< the atmosphere is
unstable & convection occurs.
• As a rising air packet A expands, it
cools slower than the surroundings
• TA(z+dz) > TATM(z+dz), making A
warmer & less dense than its
surroundings, accelerating its rise
Γd is constant
(slope = Γd)
(slope = Γd)
(slope Λ)
(slope Λ)
Λ changes depending on conditions
Little vertical mixing
Fast vertical mixing - convective
The Planetary Boundary Layer
• The subsidence inversion creates stability & inhibits mixing, often
leading to bad pollution build-up in large cities
• Planetary Boundary Height = 500 – 3000 m.
• Mixing near the surface is always fast because of turbulence
The Planetary Boundary Layer: diurnal
variations
• During the day, the earth heats the
surface layer by conduction and then
convection mixes the region above in
the convective mixed layer. There is
usually a small T inversion (dT/dz >0)
above this which marks the top of the
BL. This slows transfer from the BL to
free troposphere (FT). Traps pollutants.
• Night – surface cools, dT/dz > 0 in
surface layer – surface inversion.
Confines pollutants to surface layer.
• Can get extreme inversions in the
surface layer in winter that can lead to
severe pollution episodes. High build
up of pollutants.
Vertical Mixing
• Average atmospheric lapse rate is 6.5 K km-1, giving moderately stable
conditions
• Turbulence, most important near the surface, increases mixing
• Solar heating also makes the atmospheric unstable & increases mixing
(accounts for different mixing between night and day)
• Water vapor and clouds complicate all these things
• The stratospheric Temperature inversion significantly limits vertical
mixing between the troposphere & Stratosphere, limiting transport of
many ground level VOCs to the stratosphere (The polar regions are
special though!)
• Tropospheric/stratospheric mixing times are on the order of years!
• The Temperature profile of the stratosphere means it is much more
stable than the troposphere
Atmospheric Transport
•
•
Random motion – mixing
• Molecular diffusion
– Molecular diffusion is slow, diffusion coefficient D ~ 2x10-5 m2 s-1
– Average distance travelled in one dimension in time t is ~(2Dt)
– Molecular diffusion more important at very high altitudes & low
pressures
• Air Parcel diffusion
– In the troposphere, eddy diffusion of air parcels is more important
with a diffusion coefficient Kz ~ 20 m2 s-1
– Takes ~ month for vertical mixing (~10 km). This has implications
for short and long-lived species.
Directed motion
– Advection – winds & geostrophic flow
– Occurs on a number of different scales
• Local (e.g. offshore winds & sea breeze – see earlier)
• Regional (weather events)
• Global (Hadley circulation)
Horizontal Mixing Processes
Global circulation – Hadley Cells
• To a first approximation, horizontal mixing within the atmosphere is
well described as sea breeze circulation driven by the T difference
between the hot equator and cold polar regions
• Hadley circulation model developed in the 18th Century
Intertropical conversion zone (ITCZ) – rapid vertical transport
near the equator.
Coriolis Forces
• Longitudinal winds are well described by a coupling of Hadley type
circulation to Coriolis forces
• What is a Coriolis force?
3d example
2d example
http://www.youtube.com/watch?v=BPNLZyBNPTE&feature=related
http://www.youtube.com/watch?v=Wda7azMvabE&NR=1
Coriolis Forces & Hadley Cells
Geostrophic Flow: A balance of Coriolis
Forces & Pressure Gradients
The theoretical flow that
would result if the system
was no more complicated
than Coriolis forces and
parallel isobars
The general circulation: Hadley Cells
coupled to Coriolis Forces
Polar high pressure region
Westerlies
High pressure latitudes
(location of major deserts)
Trade Winds
(easterlies)
ITCZ
Trade Winds
(easterlies)
High pressure latitudes
(dry areas)
Stronger westerlies
Roaring 40s & Screaming 50s
(less friction)
Polar high pressure region
Ferrel cell
Horizontal transport timescales
Summary
• Atmospheric chemistry depends on atmospheric structure
& transport dynamics
• Some simple physics gives us basic insight into some of
the principles that determinate atmospheric structure and
transport dynamics
– The barometric equation describes the relationship
between pressure & altitude
– The adiabatic lapse rate helps us understand the
atmospheric vertical T dependence, and vertical
transport
• To a first approximation, global circulation may be thought
of as sets of sea breeze cells coupled to Coriolis forces
• Mixing processes are coupled to chemical change, which
we will learn about tomorrow
Some Questions to Consider
• Using your knowledge of (1) the adiabatic lapse rate and (2) the
temperature profiles of the troposphere and stratosphere, explain why
vertical mixing in the stratosphere is much slower than in the
troposphere
• Using (1) a diagram involving adiabatic lapse temperature profiles
and (2) your knowledge of stability/instability, explain the origins of the
planetary boundary layer
• Should footballers worry about Coriolis forces when they are taking a
north to south 20m free kick that travels 50 km/hr? (hint: see Jacob
equation 4-3)
• Based on the model of general circulation:
– Why are the timescales for east west transport shorter than those
for north south transport?
• Using your knowledge of Hadley transport, the Sea Breeze model,
and Coriolis forces:
1. Explain why the Arctic is so windy
2. What direction would you expect the wind to blow on the ground in
Longyearbyen?