States of Matter
advertisement

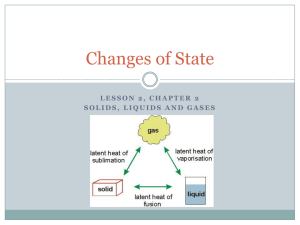
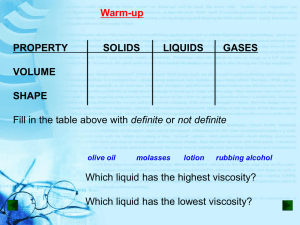
States of Matter Chapter 2 Three States of Matter Section 1 Identify the three states of matter in this scenario: You come inside from shoveling some snow. You’re cold, so you make some hot chocolate. You take a sip from the nice, steamy cup, but it burns your lip. You add an ice cube so you can drink it sooner. Identify the three states of matter in this scenario: You come inside from shoveling some snow. You’re cold, so you make some hot chocolate. You take a sip from the nice, steamy cup, but it burns your lip. You add an ice cube so you can drink it sooner. Solid: Identify the three states of matter in this scenario: You come inside from shoveling some snow. You’re cold, so you make some hot chocolate. You take a sip from the nice, steamy cup, but it burns your lip. You add an ice cube so you can drink it sooner. Solid: ice cube Liquid: Identify the three states of matter in this scenario: You come inside from shoveling some snow. You’re cold, so you make some hot chocolate. You take a sip from the nice, steamy cup, but it burns your lip. You add an ice cube so you can drink it sooner. Solid: ice cube Liquid: hot chocolate Gas: Identify the three states of matter in this scenario: You come inside from shoveling some snow. You’re cold, so you make some hot chocolate. You take a sip from the nice, steamy cup, but it burns your lip. You add an ice cube so you can drink it sooner. Solid: ice cube Liquid: hot chocolate Gas: steam Particles All matter is made of tiny particles called atoms and molecules. How the particles move determines whether an object is solid, liquid, or gas Particles are attracted to each other, but the faster the move, the more they can overcome their attraction. Solids Solids have a definite shape and volume. Particles are very close together, and there is a strong attraction between them. Each particle vibrates in place, but is locked in position by the particles around it. Kinds of Solids Crystalline solid – have an orderly, three-dimensional arrangement of particles Repeating patterns of rows. Examples – iron, diamond, ice Kinds of Solids Amorphous – particles with no special arrangement No pattern Example – glass, rubber, wax Liquids Liquids – have a definite volume, but not a definite shape. Liquids take the shape of their containers. The particles in a liquid move fast enough to overcome some of their attraction, so liquids can flow. Properties of Liquids Surface Tension A force that acts on particles at the surface of a liquid. A liquid with a high surface tension forms rounded drops. A liquid with a low surface tension forms flat drops. Properties of Liquids Viscosity A liquid’s resistance to flow A stronger attraction between particles = higher viscosity = slower flow Which has a higher viscosity: Italian dressing or ranch? Gases Gases have no definite shape or volume. Gas particles move very quickly and can break away from one another. Gases are compressible. The amount of space in between particles can change. Behavior of Gases Section 2 Temperature Temperature is the measure of how fast the particles in an object are moving. The higher the temperature, the faster the particles are moving. The faster the particles are moving, the more energy they have. Temperature A gas with a higher temperature will expand more. If you fill a balloon in cold weather, it will need more gas to be full. If it suddenly gets warm, it may pop because the gases expand. Volume We know that volume is how much space an object takes up. Because gases particles can move and spread out, their volume depends on their container. It is possible to make balloon animals because the gases in the balloon can be compressed. A water balloon would just explode! Pressure Pressure is the amount of force on an area. In gases, the pressure is the number of times the gas particles hit the inside of their container. Why is the pressure of a basketball greater than a beach ball? There are more particles of gas in a basketball than in a beach ball. Basketballs are usually filled with a pump, while beach balls are generally filled by blowing them up. Gas Behavior Temperature, pressure, and volume are linked. Changing one factor affects the other two. Boyle’s Law Boyle’s Law – for a fixed amount of gas at a constant temperature, the volume of a gas is inversely related to the pressure. Boyle’s Law This means that as the pressure increases, the volume decreases by the same amount. Charles’s Law Charles’s law – for a fixed amount of gas at a constant pressure, the volume of the gas volume and temperature change in the same direction. Charles’s Law This means that as volume increases, temperature increases. Changes of State Section 3 Changes of State ALL changes of state are physical changes. To change an state of matter, you must add or remove energy. Water is the only substance that can be found in all three states at normal surface temperature and pressure. Endothermic vs Exothermic Endothermic – energy is gained as the substance changes state Exothermic – energy is removed as the substance changes state. Melting Change of state from solid to liquid Energy is added Adding energy to a solid increases the temperature of the solid, making the particles move faster. Melting Point When a solid melts, particles must break some of the attraction that holds them together. The point where they break is the melting point. Melting point is a physical property – it could help you identify a substance. Common Melting Points What is the melting point of water? Freezing Freezing is the change of state from a liquid to a solid. Energy is removed. Removing energy makes the particles begin to lock in place. Freezing point is the time when the liquid turns into a solid Freezing Point When you put an ice cube tray in the freezer, does it freeze as soon as it touches the cold air? Why or why not? Freezing Point Does the water in the ice cube tray turn into ice all at once? Why or why not? Evaporation Evaporation is the change of state from liquid to gas. Sweat is mostly water. When you sweat, the water evaporates. You feel cooler because your body is transferring energy to the water. Evaporation Think about it: In our country, when it is hot we usually wear skimpy clothing – bathing suits, shorts, tank tops, etc. In other parts of the world with hot climates, people wear long, loose, light-colored clothing. In hot parts of the world, people also tend to eat spicy food. Brainstorm why this might be a more effective way to keep cool. Boiling Evaporation occurs at the surface of a liquid, while boiling changes a liquid to a gas all throughout the liquid. Boiling creates bubbles. The pressure inside the bubbles is called vapor pressure. Boiling occurs when the vapor pressure equals atmospheric pressure. Boiling Point The boiling point is the temperature (and pressure) at which a liquid turns to gas. This requires an input of energy, in order to break the bond of liquid particles. What is the boiling point of water? What is the boiling point of water? 212°F or 100°C Atmospheric Pressure Water boils at 100°C only at sea level. Because boiling depends on atmospheric pressure, at higher altitudes, the boiling point is lower. Ex. In Denver (in the Rocky Mountains) water boils at 95°C Condensation Condensation is the change of state from a gas to a liquid. Condensation is the reverse of evaporation, so the condensation point occurs at the same temperature as the boiling point. Condensation releases energy. Sublimation Sublimation is when a solid changes directly into a gas. This requires a high energy input to break the solid bonds. Not highly common. Carbon dioxide and arsenic often sublimate – it is difficult to find them in liquid form. Temperature and State Change Temperature is related to the speed of the object’s particles. Temperature does not change until state change is complete.