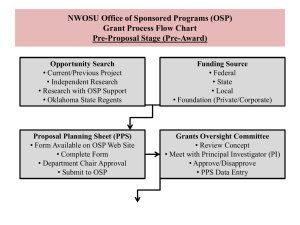
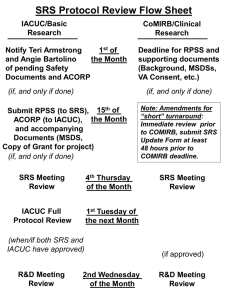
Creating Successful Research Projects: Navigating the Process
advertisement

Creating Successful Research Projects: Navigating the Process ---New Faculty Orientation--December 16, 2010 Walker Ames Room Kane Hall WELCOME David Eaton, PhD Interim Vice Provost for Research NEW UW FACULTY • 1st new faculty orientation • 375 new faculty in 2009-2010 – Transfers from other institutions – First faculty position • “…we are partners in a shared future.” (Interim President Phyllis Wise, December 2010) WORLD LEADERS IN RESEARCH We have grown into the most successful public research university in the nation in attracting support for our research. Ours is a proud culture of innovation, collaboration and discovery that has transformational impact. From the UW Mission Statement WE ARE: • World leaders in research • One of the most successful public research university in the nation in attracting support for our research • Innovative & collaborative • Impacting our community, nation and the world IMPACT • UW research makes far-reaching impacts • Locally, nationally, and globally WE ARE HERE TO HELP • Offices presenting today are part of the central oversight and support for your research enterprise. • We will work with you to ensure your projects meet sponsor requirements, state & federal law, and UW policy. TODAY’S WORKSHOP • Introduction to the central research support offices • Learn how to navigate the UW Processes • Maneuver through the multiple regulatory and compliance considerations within the grant lifecycle • Manage the pre and post award process at UW Successful acquisition & management of grants requires many partners • Individual faculty, and their departmental and Dean’s office support staff • Office of Research – all components • Grant and Contract Accounting • Management Accounting & Analysis • Human Resources • Purchasing • Facilities Management • Environmental Health & Safety • Animal Welfare OR - MAJOR ADMINISTRATIVE UNITS • Office of Sponsored Programs (OSP) Director of OSP & Assistant Vice Provost for Research, Lynne Chronister • Human Subjects Division (HSD) Director of HSD & Assistant Vice Provost for Research, Karen Moe • Office of Research Information Services (ORIS) Director of ORIS & Assistant Vice Provost, Jim Kresl • Office of Research - Central (OR) Interim Vice Provost for Research, David Eaton Associate Vice Provost-Compliance and Operations, Jeff Cheek Associate Vice Provost-External Relations, Mani Soma TODAY’S SPEAKERS • • • • • • • • • • Office of Sponsored Programs - Lynne Chronister Office of Research Information Services - Patti McClure Grant & Contract Accounting - Tami Sadusky Management Accounting & Analysis - Mike Anthony Center for Commercialization - Linden Rhoads Environmental Health & Safety - Jude Van Buren Office of Animal Welfare - Nona Phillips Human Subjects Division - Karen Moe Internal Audit - Zenaida Shattuck Conflict of Interest – Jeffrey Cheek RESOURCES AVAILABLE • A resource table is set up at the back of the room. Please stop by and collect materials you feel can assist you in creating successful research projects. • This PowerPoint deck will be posted on the OSP website tomorrow. • Evaluation form – please tell us how we did! Lynne Chronister, Assistant Vice Provost for Research & Director OSP OFFICE OF SPONSORED PROGRAMS (OSP) OSP MISSION The Office of Sponsored Programs, a unit in the Office of Research, supports and advises the entire academic community in securing external support for sponsored projects and collaborations. We review, negotiate, approve, and provide administrative oversight related to proposals and establishment of awards on behalf of the University of Washington in accordance with all applicable policies, and regulations. OSP RESPONSIBILITIES What do we do? • • • • • • Review and approve proposals Submit electronic proposals Negotiate and sign awards and subcontracts Liaison with sponsor for award life Coordinate close-out of awards Implement Grant & Contract policies (financial, legal, and regulatory) • Provide customer support to UW faculty and staff OSP STRUCTURE • Each unit on campus assigned to Team A or Team B • Team C reviews all Industry-Sponsored Clinical Trials • Subcontracts Team manages all outgoing subcontracts and required reporting • Team Structure: – Administrator – Grant & Contract Coordinator – Program Coordinator GIM 19 Business Sections • • • • • • • Face Page List of Personnel Biosketches Budget Budget Justification Checklist Required Additional Information Scope of Work • Description of the Research • (The Science) Final Version by Day – 7 Draft Version by Day – 7 Final Version by Day - 3 PROPOSAL BUSINESS PROCESS 1. Identify sponsor & complete application Hints: read & follow instructions, contact your OSP admin if you have questions 2. Fill in SAGE eGC1, attach application & route for internal approvals 3. Review & approve by OSP OSP will work with PI & Dept to ensure the best application can be submitted to the sponsor 4. Submit application per sponsor instructions o o Application is submitted by OSP/PI Via electronic, paper, web formats AWARD BUSINESS PROCESS • Sponsor may notify PI or OSP that application is fundable • Award documents arrive • OSP negotiates & accepts award (exceptions) • OSP creates eFA and sends to GCA ADMINISTRATIVE BUSINESS PROCESS • Advanced Budgets (if allowed by sponsor) • Modification to an award (PI, dates, re-budgeting, etc.) must be processed by OSP and may require sponsor approval • No-Cost Extensions • Sub-Awards are made through OSP SPONSORED PROGRAM POLICY GIMs (Grant Information Memoranda) • Provide policy and guidance on submitting and managing awards • Linked from the OSP Home page • Currently “Under Construction” OSP HINTS • Contact your OSP Administrator early in the process with questions. • What does the PI sign? Nothing that will create liability or commitment • What does OSP sign? Anything that commits the institution New Facilities and Administrative (F&A) Rates Location Negotiated Rates (FY 2010-2014) On-campus 54% -- FY 2010-2012 54.5% -- FY 2013-2014 Off-campus 26% South Lake Union 66% -- FY 2010; 68% FY 2011; 72% -- FY 2012; 73% -- FY 2013; 74% -- FY 2014 Regional Primate Center 42% (A)/ 78% (A+B)/ 83% (A+B+C) Applied Physics Lab 17% Other Sponsored Activity 33.8% (on-campus) 26% (off-campus) Vessel 25% (S&W) Instruction 53.0% (on-campus) 26% (off-campus) OSP RESOURCES • Grants Information Memoranda (GIM) • OSP Web Page • Researcher’s Guide • Monthly Research Administration Meetings (MRAM) o Subscribe to List Serve - get meeting announcements & email updates o MRAM Web site o Monthly in-person updates to campus CLINICAL RESEARCH • School of Medicine – Clinical Research • Richard Meisinger, PhD, Ass’t Dean for Planning and New Initiatives, Office of Research and Graduate Education, School of Medicine, rjmjr@uw.edu • Ella Mae Kurashige, Director, Clinical Research Services, ellamaek@uw.edu • Office of Clinical Research Budget & Billing (CRBB) • Clinical Trial start-up and web resources Patti McClure – Educational Outreach Specialist Tiffany Austin – Learning Specialist OFFICE OF RESEARCH INFORMATION SERVICES (ORIS) OFFICE OF RESEARCH INFORMATION SERVICES (ORIS) Serving UW's research community through IT solutions Web Services Data Reporting SAGE System to Administer Grants Electronically Research Road Map Hosted Services SAGE (System to Administer Grants Electronically) • Create, route, and manage the electronic routing form (eGC1) required for all UW grants and contracts/research applications • Centrally store and share grant proposal information • Access grant proposal information from any internetconnected location WHEN TO USE SAGE? Activity in SAGE The Idea Generate the idea Identify potential sponors Synthesize idea with sponsor's mission Begin drafting proposal Consider budget The Application Create an eGC1 Create a budget Complete proposal & gather documents Upload final documents to eGC1 Route eGC1 for approval Submit to sponsor The Award Confirmation from sponsor Request Advance Budget Gather Just in Time approvals Receive funding Manage your award The Research Conduct research Report findings Generate new ideas SAGE BUDGET An easy-to-use budget worksheet customized to comply with Research and UW Accounting Rules • Supports UW object code accounting • Provides up-to-date salary & benefits information • Identifies correct F&A rate(s) for your budget • Attaches to eGC1 eGC1 APPROVALS • Routes your eGC1 to the appropriate parties for online review and approval • Enables you to check on routing status of your eGC1 GET STARTED WITH SAGE • Request access from your department • Practice on the training server • Enroll in SAGE 101 training course www.sage.washington.edu CONTACTS & QUESTIONS • SAGE Learning – Email: orislearn@uw.edu • SAGE Help Desk – Email: sagehelp@uw.edu – Telephone: 685-8335 • Questions? Tami Sadusky, Executive Director GRANT AND CONTRACT ACCOUNTING/EQUIPMENT INVENTORY OFFICE Division of Research Accounting & Analysis within Finance & Facilities Finance & Facilities RESEARCH ACCOUNTING & ANALYSIS (RAA) RAA Mission: We help people who change the world Research Accounting & Analysis (RAA) Grant & Contract Accounting (GCA) Equipment Inventory Office (EIO) Management Accounting & Analysis (MAA) GCA RESPONSIBILITIES What do we do? • Set up grant and contract budgets in the University’s Financial Accounting System (FAS) • Prepare and submit required invoices and financial reports to sponsoring agencies • Manage grant and contract cash transactions and payments • Provide oversight and guidance on compliance issues • Coordinate financial close-out of budgets • Monitor grant related transactions on a sampling basis • Work with Internal Audit on all grant related audits • Provide customer support to UW faculty and staff EIO RESPONSIBILITIES • • • • • • • • Prepare Closing Inventory Reports Facilitate State, Federal, and Agency inventories Assist departments with equipment fabrications Print and distribute asset tags to departmental inventory contacts Train departments on General Inventory Procedures, OASIS Access, and Inventory processing Assist PI’s taking equipment out of the country Review and approve transfers of equipment out of the University Facilitate annual depreciation for the University’s financial statements GCA/EIO Structure • ‘Streams’ of Fiscal Analysts handle core processes: – Budget set up – Reporting - Invoicing - Closing • Cash Team handles grant related cash transactions and applications • Grant Analysts handle all campus communication including outreach and training • Complex Grant Analysts handle complex grants and contracts • Equipment Team handles all equipment processes POST AWARD PROCESS Proposal & Award Process Academic Dept/Office of Sponsored Programs Assignment of Budget Numbers Receipt and processing of eFA (Electronic Funding Action) Active Grant Period Expenditure Allowability Payment requests to sponsors Closing Fiscal Reporting External to Grant and Contract Accounting Audits AWARD LIFECYCLE Award Step Identify funding Prepare proposal Review and submit proposal Negotiate and accept award Set up award Perform work Financial management Technical reporting Monitor activity Invoice and collect payments Monitor accounts receivable Close-out award Responsible Party PI PI & Department Staff PI & Office of Sponsored Program (OSP) OSP Grant & Contract Accounting (GCA) PI PI, Department Staff, GCA PI PI & Department Staff GCA GCA & Department Staff PI, Department Staff, GCA & OSP THE BUDGET • Provides the framework for financial compliance • GCA creates a budget number for an award to accumulate costs associated with the project. • The budget is allocated in the financial accounting system (FAS): – Direct costs – Facilities and Administration (F&A or indirect costs) DIRECT COSTS Costs that should be directly charged to sponsored agreements when they can be specifically identified to the work performed under those agreements. - Salaries, Wages, and Fringe Benefits - Supplies and Materials - Equipment INDIRECT COSTS Cost that are normally considered administrative, infrastructure or benefit multiple projects – Administrative and Clerical salaries – Basic local phone service – Office supplies – Routine copying charges POLICIES Federal, State, University & Sponsor Award Terms Departmental Policies Learn How to Apply the Policies Accounting & Financial Policies University Policies & Procedures NIH - GPS A-21, A-110, FARs Sponsor Policies Federal Policies and Governmental Law PI/DEPARTMENT RESPONSIBILITIES • • • • • • • • • Assistance in grant and contract reporting/closings Separation of (high risk) duties, such as payroll or petty cash Deficit resolution Program Income Cost Sharing/Faculty Effort Reporting Unexpended balances Records retention Faculty Effort Certifications (FECs) Address problems related to fiscal activities promptly HINTS • Take a proactive approach to grant management • It’s critical that all parties work together • Adhere to University, State, Federal and Sponsor policies and regulations • Establish proper financial accountability structure • Ensure proper financial management with attention to data integrity GCA RESOURCES • GCA Web Page • GCA email/phone: gcahelp@u.washington.edu 206-543-8454 • Grant Tracker • Researcher’s Guide • Monthly Research Administration Meetings (MRAM) • Grants Information Memoranda (GIMs) Michael Anthony, Executive Director MANAGEMENT ACCOUNTING & ANALYSIS (MAA) Division of Research Accounting & Analysis within Finance & Facilities Finance & Facilities RESEARCH ACCOUNTING & ANALYSIS (RAA) RAA Mission: We help people who change the world Research Accounting & Analysis (RAA) Grant & Contract Accounting (GCA) Equipment Inventory Office (EIO) Management Accounting & Analysis (MAA) MAA – OFFICE RESPONSIBILITIES What do we do? • Calculate and negotiate the University’s Facilities and Administrative (F&A) rates • Review and approve Recharge Center/Cost Center/Program Income rates • Provide oversight for the Federally mandated effort reporting process • Provide support to UW faculty and staff MAA - STRUCTURE Major Department Activities/MAA Units: • F&A rate calculation and negotiation • Recharge/cost center and program income rate review and approval • Effort reporting training and oversight All units participate in customer service to faculty and staff F&A RATE CALCULATION & NEGOTIATION F&A (Indirect) Costs • Infrastructure costs necessary to support the UW’s research mission • Represents ‘real’ institutional costs • Incurred for common or joint objectives • Cannot be identified specifically with a sponsored project or other institutional activity • Recovered through the application of the F&A rate to funded sponsored agreements CURRENT F&A RATES (effective 7/1/09) • 54% On-campus Research • 26% Off-campus • 33.8% “Other Sponsored Projects” (New rate for UW will be used for non-research and non-training or education) • Other special rates (e.g., Applied Physics Lab; South Lake Union; Regional Primate Center) • http://f2.washington.edu/fm/maa/fa/rates RECHARGE /COST CENTERS • Recharge/Cost Centers - units or activities providing goods/services primarily to internal university operations • Recharge Centers - charge more than $125,000 annually to federally sponsored agreements OR more than $1,000,000 in total charges • Cost Centers - charge less than $125,000 annually to federally sponsored agreements AND less than $1,000,000 in total charges PROGRAM INCOME • Income generated as a result of a research activity • Tied to a specific grant or contract, usually Federally funded • Income may be internal and/or external • These types of activities require prior approval from the agency that sponsored the original research PROGRAM INCOME RATES • MAA – Reviews rates to ensure they are compliant with Federal costing principles – Verifies costs are not being recovered through other means, e.g., through the F&A rate process • GCA – Establishes program income budgets – Ensures program income is netted from reimbursement requests EFFORT REPORTING • Federally mandated compliance requirement • Requires periodic certification that percent salary charged to sponsored project represents a reasonable reflection of percent effort devoted to the project • Includes committed cost shared effort • Faculty – Accomplished through Faculty Effort Certification (FEC) • Non Faculty – Accomplished via Grant and Contract Certification Report (GCCR) EFFORT REPORTING • Faculty Effort Certification (FEC) – Done every 6 months – SOM/Dentistry/Public Health/Pharmacy • January 1 – June 30 • July 1 – December 31 – All other schools/colleges • September 16 – March 15 • March 16 – September 15 • Grant and Contract Certification Report (GCCR) – Done on the calendar quarter MAA - RESOURCES • GIM (Grants Information Memoranda) – GIM 13: Indirect Cost Rates – GIM 22: Facilities and Administrative Costs: An Explanation – GIM 35: Effort Reporting Policy for Sponsored Agreements • MAA Web Page • MRAM (Monthly Research Administrators Meeting) Linden Rhoads, Vice Provost CENTER FOR COMMERCIALIZATION (C4C) Jude Van Buren, Director ENVIRONMENTAL & OCCUPATIONAL HEALTH & SAFETY (EH&S) EH&S - MISSION To support the University of Washington’s teaching, research, and service missions, the Environmental Health and Safety Department assists organizational units in meeting their responsibility to: – protect the environment – provide a safe and healthful place of employment and learning EH&S Functional Org Chart How we are organized to support you Conducting UW research has many parallels to running a successful business • You have employees to protect and supervise • You have materials (biologicals/ chemicals/ radiation) • You may have animals or plants under your care • You have procedures, methods and protocols for your processes • You have approvals and laws that govern your practices • You have a facility you need to maintain • You have neighbors you must consider Local, State and Federal laws that address your research: EH&S has responsibilities and authority provided in UW Admin. Policy Statements (APS) to meet those regulations State and Federal Environmental Pollution Laws State and Federal Worker Health & Safety Laws State and Local Public Health /Fire Safety Laws UW APS & Your Research Hazardous materials laws Federal sponsor requirements Fed. laws: Select Agent, Agriculture, Animals Hospital Operational/ Facility Laws 5 questions on SAGE that will engage EH&S involvement in your research 1. EHS-1: Any Pathogenic Agents, Potential Biohazards, recombinant DNA, human tissues or cells, hazardous materials in animal studies or highly toxic chemicals? If YES – then: 2. EHS-2: Any involvement of acquisition, possession, use transfer or shipping of Select Agents, Exempted Select Agents or Toxins? 3. EHS-3: Generate hazardous waste without disposal options or mixed waste (radiation + Hazardous components) or multi-hazard waste (biological /hazardous /radiological components)? 4. EHS-4: Involvement of uses of radiation: transuranics, gaseous alpha-emitters or intentional release of radionuclides to atmosphere? 5. EHS1-A: Does Sponsor require Institutional (UW EH&S) Safety Review of facility prior to submission to the sponsor. MUST HAVES – for working with biological material and/or recombinant DNA 1. Biological Use Authorization (BUA) Institutional approval from EH&S and the Institutional Biosafety Committee (IBC). All work with Recombinant DNA, pathogenic organisms, human or non human primate tissues (including all cell lines) or other biohazardous agents. http://www.ehs.washington.edu/rbsresplan/rpha.shtm 2. Biological Safety Manual use required and made available to staff http://www.ehs.washington.edu/rbsbiosafe/bsmanualindex.shtm 3. Biological Safety Training required by NIH for all of above research prior and annually for work with biohazards In person class offered monthly – soon to be on-line 4. Bloodborne pathogen training (BBP) required when working with human blood, blood byproducts and human tissue – (new and annual trainings – in person and soon on-line) ALL EH&S trainings can be found on our webpage. MUST HAVES – for working with animals and/or hazardous materials Occupational Health Reviews: • All researchers and staff working with Animals • Must complete EH&S Animal Use Medical Screening (AUMS) Form - receive medical clearance. Review required prior to initiation (IACUC approval) and every 3 years. • All Animal Research using hazardous materials • IACUC approval requires Occupational Health Nurse (OHN) review of research involving biohazards, chemical hazards • OHN sends PI letter and IACUC recommendations about these hazards in their protocol • PI must review OHN recommendations with staff and adhere to vaccinations requirements where indicated and work safety practices including protocols and personal protective equipment • Highly hazardous chemicals are referred to EH&S Occupational Hygienist for follow up – possible lab visit and changes to protocols and SOPS to address hazards MUST HAVE – engineering controls when working with chemicals • Appropriate room ventilation • Functional safety shower and eye wash • Fume hood with recent EH&S performance test • Appropriate chemical storage cabinets • Spill kits/secondary containment • Appropriate ventilation if using chemicals in a certified biosafety cabinet MUST HAVE administrative controls for chemical use • Chemical management training • Health Hazards from exposures • Operations • Use/storage/Personal Protective Equipment • Waste/ Spills • Standard Operating Procedures • Chemical inventory, MSDSs (material safety data) and emergency contacts (MyChem) • Training for shipping hazardous materials • Fire Department hazmat permit • EH&S approval for toxic gases • All EH&S trainings at http://www.ehs.washington.edu/psotrain/corsdesc.shtm#initialbloodborne If you are working with radiological materials you MUST have: • Been granted Authorized Investigator (AUI) status by the EH&S Radiation Safety Office or be working under an existing AUI. • X-ray and laser generating equipment must registered with the Radiation Safety Office. • Training must be verified and inspection schedules established for x-ray producing equipment. • Disposal of these items must be done properly. Users must notify the RSO prior to disposal. As a business person you have employees that you must protect, mentor and govern Responsibilities you must address for your employees welfare: 1. Report employee accidents Employee must inform boss and EH&S http://www.ehs.washington.edu/ohsoars/index.shtm 2. Inform employees of what they are working with: Worker Right-to-Know Communications http://www.washington.edu/admin/rules//APS/12.05.html Identify & label hazardous chemicals appropriately Access Material Safety Data Sheets (MSDSs) http://www.ehs.washington.edu/epomychem/msds.shtm 3. Review current Lab Safety Manual, keep it in your lab & available to staff http://www.ehs.washington.edu/manuals/lsm 4. Report chemicals used in MyChem – chemical inventory database http://www.ehs.washington.edu/epomychem/index.shtm As a business person you have employees that you must protect, mentor and govern 5. Identify hazardous procedures and describe employee protective measures “Chemical” Lab http://www.ehs/washington.edu/manuals/lsm/lsm6.pdf “Physical” Lab http://www.ehs.washington.edu/rbsresplan/ppe.shtm 6. Know your Emergency Procedures/Plans Ask your Department for their Health and Safety Plans and your building’s Emergency Evacuation and Operations Plan 7. Staff Training- required trainings plus trainings to educate new researchers and staff Ensure all training is documented, keep training current, train all new staff http://www.ehs.washington.edu/manuals/lsm/lsm7.pdf Operating a Research Lab is like running a business – What are the risks? • Fines/Restrictions • Health Impacts • Unable to do research/Negative Publicity • UW Mission at risk Examples of costs of not following procedures/protocols/regulations • Failure of researcher to properly dispose of hazardous waste (>$10,000 fine -2010) • Violation of air operating permit from EPA resulted in a $350,000 fine (Negotiated to $30,000 fine with program initiation - .5 EH&S FTE) • Pending appeal of $66,000 fine to UW for explosion and violation of FAA shipping regulations (2010) What YOU can do to ensure accidents, injuries, citations, fines and loss of research doesn’t occur It depends on you---• Know and follow the policies • Know SOPs • Prepare and practice • Protect self & employees • Perform compliance Checks • Promote worker health and safety and environmental stewardship Questions? Contact EH&S at 206.543.7262 or at ehsdept@uw.edu Nona Phillips, Director, Office of Animal Welfare OFFICE OF ANIMAL WELFARE (OAW) Office of Animal (OAW) - Mission The Office of Animal Welfare provides support to the University’s Institutional Animal Care and Use Committee (IACUC) and provides support and advice to researchers utilizing live vertebrate animals for teaching and research. We review IACUC protocols, grants involving live vertebrate animals, and provide oversight and compliance on behalf of the IACUC in accordance with applicable laws, policies and regulations. OAW - RESPONSIBILITIES What do we do? • Process and review IACUC protocol submissions • Review grants and provide IACUC approval information for sponsors • Provide courses including species-specific procedure labs • Provide personal one-on-one post-approval assistance to researchers via our “Post-Approval Monitoring” program • Coordinate IACUC semi-annual site visits to animal housing and use locations OAW - STRUCTURE Administrative Team: Processes IACUC submissions and informs PIs of upcoming protocol and training expirations. Scientific Reviewer Team: Reviews IACUC submissions and grants involving live animals. Training Unit: In conjunction with Department of Comparative Medicine veterinary staff, courses are provided including species-specific labs, surgery, etc. Site Visit Team: Coordinates all aspects of IACUC semi annual site visits and tracks deficiencies and corrections. Post-Approval Monitor Team: Each PI is assigned to an individual who will meet with them to review protocols and plans, and advise/assist them as needed. IACUC Protocol Business Process 1. PI submits new IACUC protocols, renewals, and changes to protocols. 2. IACUC members receive items semi-monthly. 3. Members have 1 week to review and submit questions/comments. (Members may request Full Committee Review of any item) 4. IACUC questions/comments forwarded to PI. 5. PI responds back to committee with answers & protocol revisions. IACUC Protocol Business Process 6. OAW scientific reviewer reviews PI answers/ protocol revision. 7. Reviewer works with PI on any outstanding issues and then either: • approves the protocol or • assigns it for the next monthly convened meeting if Full Committee Review was/is requested by any IACUC member. Grants & Contracts • Competing proposals – Just-in-Time (after submission to sponsor but prior to award) – Copy of the proposal + eGC1 • Non-competing proposals – At time of proposal submission – Automatically routes to OAW during SAGE eApprovals • IACUC approval info must be included for animal work at other institutions, if applicable. Grant/Contract Review Business Process 1. OAW reviews grant and associated UW protocol(s) to assure concordance and if applicable, assures that approvals from other institutions are documented. 2. OAW provides letter to sponsor re IACUC approval based on sponsor requirements. 3. Other documents provided as required by the sponsor. TRAINING OPPORTUNITIES • Courses are offered on-line and in-person • Courses include: – general laws and regulations – species-specific labs – facility orientations • Training specific to non-human primates is offered by the Washington National Primate Research Center • See the Animal Use Training web site for details and registration instructions POST APPROVAL MONITORING • Each research group is assigned a compliance advisor. • The compliance advisor meets once or twice yearly with the PI (and lab manager and/or others per PI’s preference). • Visits include review of protocols, operating procedures, and planned changes, and advice regarding IACUC review requirements. IACUC SITE VISIT – BUSINESS PROCESS 1. The IACUC conducts semi-annual site visits to animal housing and use rooms during January-April and JulyOctober. 2. OAW requests available times, in advance, from research groups and then schedules a visit accordingly. 3. The PI or facility supervisor receives a written report after the visit. 4. If there are deficiencies a reply with correction plan and completion date(s) is required. IACUC POLICIES Policies reviewed and approved by the IACUC can be viewed and/or downloaded. HINTS FOR SUCCESS • Contact OAW whenever you have questions regarding your use of animals, including when you may be unsure of whom to contact. • We will help you or refer you to the appropriate office or department. Consult with OAW when… • Changing the scope of grants (i.e., adding use of animals when that was not originally planned and approved by the sponsor, or adding a foreign component). • Contracting with outside institutions/ companies for animal work (e.g., custom antibodies) to assure that an acceptable institution is chosen. • When consulting with OAW regarding either of the above, please visit our website and e-mail a scientific reviewer. OAW RESOURCES • OAW/IACUC Web Page https://depts.washington.edu/iacuc • Animal Use Training Web Page http://depts.washington.edu/auts/index.html Karen Moe, Asst Vice Provost for Research & Director HSD HUMAN SUBJECTS DIVISION (HSD) HSD - MISSION The Human Subjects Division (HSD) is a unit within the Office of Research (OR). HSD staff support and facilitate the review of human subjects research by the Institutional Review Board (IRB). HSD staff and IRB members partner with researchers to safeguard the rights and welfare of human subjects in UW research. HSD - RESPONSIBILITIES What do we do? – Provide oversight, administrative support, and assistance for research subjects and for researchers – Support the IRBs in their review of research – Make determinations about whether an activity is human subjects research, or qualifies for exempt status – Post-approval monitoring – Assure compliance with federal regulations, state laws, and university policy governing human subjects research – Develop, deliver, and facilitate access to education and training in the protection of human research subjects HSD - STRUCTURE 3 Biomedical IRBs: 3 Social/Behavioral IRBs: 1 Combined IRB: 4 Subcommittees: (Subcommittees review research that is Exempt or Minimal Risk.) REVIEW BY NON-UW IRBs • Western IRB (WIRB) Reviews industry-sponsored and -initiated clinical trials. • Cancer Consortium IRB (CC-IRB) Reviews cancer research from consortium members. • Cooperative Agreements (Affiliated Institutions) Reviews conducted by Seattle Childrens, Group Health, Swedish Hospital, etc. and accepted by the UW IRB. IRB BUSINESS PROCESS IRB APPROVAL CRITERIA 1. Risks are reasonable relative to benefits Anticipated benefits include the importance of the knowledge that may be expected to result. 2. Risks to subjects are minimized • Procedures are consistent with sound research design and do not unnecessarily expose subjects to risk. • If possible, the research uses procedures already being performed on subjects. 3. Protection of subject privacy & confidentiality There are adequate provisions to protect the privacy of subjects and to maintain the confidentiality of data. 4. Adequate safety monitoring plan The research plan has adequate provisions for monitoring the data collected, to ensure the safety of subjects. IRB APPROVAL CRITERIA 5. Written informed consent obtained From each prospective subject or the subject’s legally authorized representative, unless the IRB, per federal criteria: – Waives the need for written consent. – Waives the need for consent. 6. The consent process provides all required and appropriate information Unless waived by the IRB per federal regulatory criteria. 7. Subject selection is equitable Taking into account: – The research purpose and setting, and – Any special issues associated with vulnerable populations. IRB APPROVAL CRITERIA 8. Additional safeguards for protected & vulnerable populations – Additional safeguards are included to protect subjects who may be vulnerable to undue influence or coercion. – Includes: children, prisoners, pregnant women, neonates. – May also include decisionally-impaired, or economically or educationally disadvantaged subjects. 9. Other ethical & compliance issues – Researcher conflict of interest. – Involvement of non-UW institutions and individuals. – Other compliance requirements. HINTS FOR SUCCESS • Allow sufficient time for both preparation of the IRB application and its review. • Go to the HSD website to download a new application each time (things may have changed). • Don’t leave anything blank on the application – at least write-in NA, as appropriate. • Contact HSD with your questions or as issues arise . . . we’re here to help. HSD RESOURCES • HSD Website: • Get Started >> The IRB Process & IRB Guides • Policy & Procedures • Announcements • eNews from HSD and the IRB (please subscribe) • Contact HSD: • hsdinfo@uw.edu – general inquiries • hsdforms@uw.edu – question or suggestion with a form • hsdtrain@uw.edu – request assistance or a session Zenaida Shattuck, Associate Director INTERNAL AUDIT DEPARTMENT WHO AUDITS YOU? • Federal and state auditors • Private/non-government sponsors • Local government auditors • Financial statement auditors • Internal Audit WHEN AN AUDITOR CALLS • Determine the purpose of the call • Immediately notify your department administrator • If the auditor asks for information, request for time to consult with staff WHEN THE AUDITOR ARRIVES You may have a University representative present when meeting with an auditor. – School/Department Administrator – Internal Audit Liaison WHEN THE AUDITOR ARRIVES –Make sure both you and the auditor understand the audit objective. –Make sure you understand the question. –Answer honestly and openly. WHEN THE AUDITOR ARRIVES Limit conversation to internal controls and: –How you are directly involved with your grant –How expense benefited the grant –Do not bring in funding issues or departmental politics SUGGESTIONS FOR PIs • Understand state and federal guidelines • Know who is tracking your grant activity • Review and initial paperwork on a regular basis • Know your department administrator and the Internal Auditor and ask for help • Remember, you can ask for time to consult with the staff responsible for the work INTERNAL AUDIT CONTACTS Richard Cordova rcordova@uw.edu Zenaida Shattuck zshatt@uw.edu Peter Harris ph3@uw.edu Jeffrey Cheek, Associate Vice Provost for Research Compliance& Operations, Office of Research Conflict of Interest (COI) FEDERAL RESEARCH STANDARD Conflicts of financial interest occur “whenever financial considerations may have the potential to compromise or have the appearance of compromising an investigator’s professional judgment and independence in the design, conduct, or publication of research.” - Public Health Service Potential conflicts of interest occur in all human processes and are not inherently “bad” if they are appropriately managed. COMMON FINANCIAL INTERESTS • Money – cash, salary, fees, royalties, honoraria; any monetary right or obligation (both creditors and debtors have monetary interests), liabilities • Property – any physical asset with monetary value or burden; intellectual and intangible property • Equity/ownership – stock, partnership, etc. • Imputed interests – spouse, family, partnership, joint ventures, other legal relationships Bottom Line: Anything with economic value UW SIGNIFICANT FINANCIAL INTEREST DISCLOSURE POLICY (GIM 10) • Applies to all research (sponsored and unsponsored) and tech transfer license transactions • Addresses conflicts and appearances of conflicts • Goal is to prevent • Bias in research • Harm to human subjects • Misuse of UW and state resources • Violations of state ethics act GIM 10 Purpose: • Ensure no research or tech transfer activities at UW are adversely affected by outside financial interests of persons involved in those activities • GIM 10 Policy complies with PHS and NSF requirements for policy pertaining to financial conflicts of interest of research investigators GIM 10 Disclosure: • Prior to participating in research or tech transfer activity, anyone having a significant financial interest related to the activity must disclose details • Can occur: when research proposal submitted to OSP; when application submitted to HSD; when SFI arises during the course of research; or prior to concluding technology licensing transaction TYPICAL SIGNIFICANT FINANCIAL INTERESTS (SFI) • Outside salary, honoraria, consulting fees • Compensation for speaking engagements • Stock, stock options, other ownership interests • Intellectual property rights (patents, licenses) • Invention royalties (UW distributed royalties and equity not considered SFI for license transactions) • Imputed interests (spouse, etc.) UW DEFINITION OF “Significant Financial Interest” 1. For a Clinical Trial, any Financial Interest. 2. For Human Subjects Research other than a Clinical Trial (i) any Financial Interest exceeding $5,000 in value, (ii) any Equity Interest; or (iii) any Intellectual Property Interest. 3. For all Research other than Human Subjects Research and all Technology Transfer Transactions, (i) any Financial Interest (including a Compensation Interest, an Equity Interest and an Intellectual Property Interest) exceeding $10,000 in value, or (ii) any Equity Interest representing more than a 5% ownership in any single entity. (PHS/NSF definitions) NOTE: PHS/NIH currently revising guidelines (and perhaps definitions of SFI); new regulations and/or guidance anticipated by Fall of 2011. UNIVERSITY OUTSIDE PROFESSIONAL WORK POLICY • Pre-approval required (chair, dean, Office of the Provost) • Subject to pre-approval, faculty may engage in outside work for compensation • Policy (and accompanying form) requires disclosure of days and nature of work, not compensation (not true for SOM faculty) www.washington.edu/admin/acadpers/forms/approval_compensation.pdf • Approval for up to 13 days per quarter • Annual report listing all outside professional activities, whether or not compensated, must be filed OUTSIDE WORK SUMMARY • UW policy supports approved outside work • Advance approval required • Form not required for some non-profit work • Requests involving potential conflicts of interest are scrutinized • SoM Supplement form required • De minimis use of UW resources permitted 2005 STATE ETHICS ACT CHANGES - Effect • Alternate compliance system for “University Research Employees” at state universities (research faculty/employees engaged in research/tech transfer – not other employees) • Universities allowed to adopt administrative processes, with the approval of the Governor, that apply in place of obligations otherwise imposed by Ethics Act • Ethics board retains authority to enforce violations of alternative compliance system • Liberalization of permitted de minimis uses • See our FAQs on Office of Research website LIMITATIONS TO KEEP IN MIND 1. Faculty who own 50% or more of a company may be required to take a whole or partial leave to do work for it. 2. Faculty member cannot be an investigator in a clinical trial involving faculty member’s invention or product. 3. The UW will not conduct a clinical trial of a UW invention or for a company in which it has a substantial equity position. 4. Except for allowed de minimis uses, UW resources and facilities can only be used to support a company through established processes (sponsored research, contracts, etc). 5. UW intellectual property cannot be transferred through consulting or other “backdoor” methods. Lynne Chronister, Assistant Vice Provost for Research & Director OSP Wrap-Up UNIVERSITY OF WASHINGTON • Leaders in research and education • Positively impacting our community, nation and the world • It Takes a Village: – We are all partners in this successful research enterprise TODAY’S WORKSHOP • Introduction to the central research support offices • Learn how to navigate the UW Processes • Maneuver through the multiple regulatory and compliance considerations within the grant lifecycle • Manage the pre and post award process at UW WE ARE HERE TO HELP • Offices presenting today are part of the central oversight and support for your research enterprise. • We will work with you to ensure your projects meet sponsor requirements, state & federal law, and UW policy. • Contact us with your questions. RESOURCES AVAILABLE • A resource table is set up at the back of the room. Please stop by and collect materials you feel can assist you in creating successful research projects. • This PowerPoint deck will be posted on the OSP website tomorrow. • Evaluation form – please tell us how we did! THANK YOU