Coculture With Embryonic Stem Cells Improves Neural
advertisement
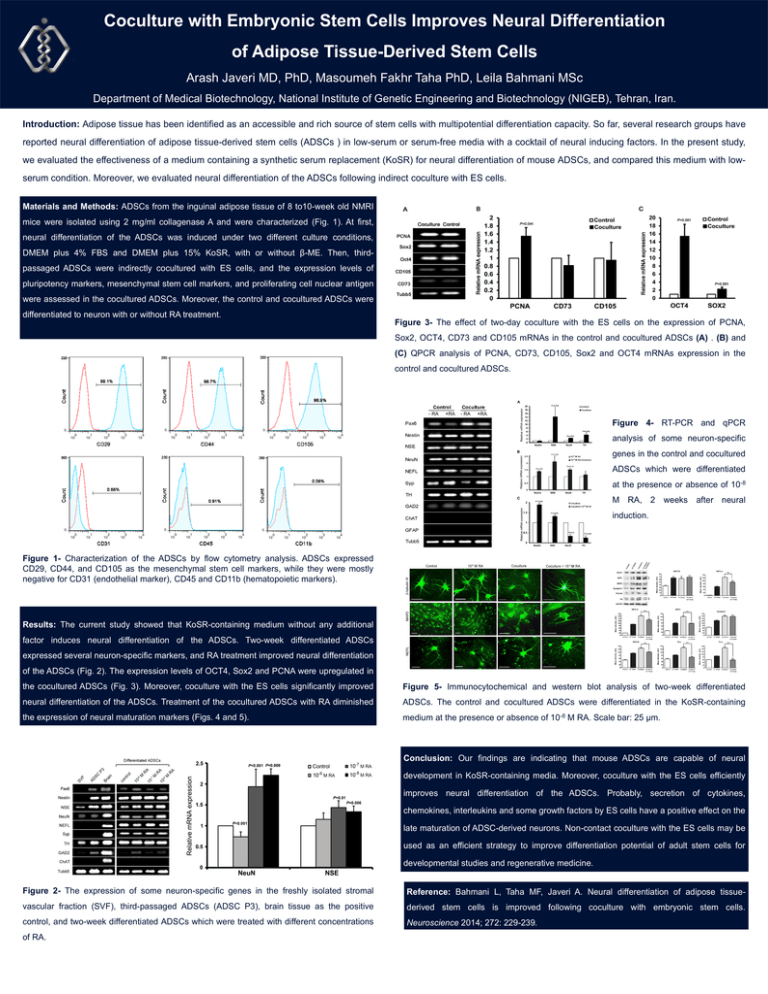
Coculture with Embryonic Stem Cells Improves Neural Differentiation of Adipose Tissue-Derived Stem Cells Arash Javeri MD, PhD, Masoumeh Fakhr Taha PhD, Leila Bahmani MSc Department of Medical Biotechnology, National Institute of Genetic Engineering and Biotechnology (NIGEB), Tehran, Iran. Introduction: Adipose tissue has been identified as an accessible and rich source of stem cells with multipotential differentiation capacity. So far, several research groups have reported neural differentiation of adipose tissue-derived stem cells (ADSCs ) in low-serum or serum-free media with a cocktail of neural inducing factors. In the present study, we evaluated the effectiveness of a medium containing a synthetic serum replacement (KoSR) for neural differentiation of mouse ADSCs, and compared this medium with lowserum condition. Moreover, we evaluated neural differentiation of the ADSCs following indirect coculture with ES cells. Materials and Methods: ADSCs from the inguinal adipose tissue of 8 to10-week old NMRI mice were isolated using 2 mg/ml collagenase A and were characterized (Fig. 1). At first, neural differentiation of the ADSCs was induced under two different culture conditions, DMEM plus 4% FBS and DMEM plus 15% KoSR, with or without β-ME. Then, thirdpassaged ADSCs were indirectly cocultured with ES cells, and the expression levels of pluripotency markers, mesenchymal stem cell markers, and proliferating cell nuclear antigen were assessed in the cocultured ADSCs. Moreover, the control and cocultured ADSCs were differentiated to neuron with or without RA treatment. Figure 3- The effect of two-day coculture with the ES cells on the expression of PCNA, Sox2, OCT4, CD73 and CD105 mRNAs in the control and cocultured ADSCs (A) . (B) and (C) QPCR analysis of PCNA, CD73, CD105, Sox2 and OCT4 mRNAs expression in the control and cocultured ADSCs. Figure 4- RT-PCR and qPCR analysis of some neuron-specific genes in the control and cocultured ADSCs which were differentiated at the presence or absence of 10-8 M RA, 2 weeks after neural induction. Figure 1- Characterization of the ADSCs by flow cytometry analysis. ADSCs expressed CD29, CD44, and CD105 as the mesenchymal stem cell markers, while they were mostly negative for CD31 (endothelial marker), CD45 and CD11b (hematopoietic markers). Results: The current study showed that KoSR-containing medium without any additional factor induces neural differentiation of the ADSCs. Two-week differentiated ADSCs expressed several neuron-specific markers, and RA treatment improved neural differentiation of the ADSCs (Fig. 2). The expression levels of OCT4, Sox2 and PCNA were upregulated in the cocultured ADSCs (Fig. 3). Moreover, coculture with the ES cells significantly improved Figure 5- Immunocytochemical and western blot analysis of two-week differentiated neural differentiation of the ADSCs. Treatment of the cocultured ADSCs with RA diminished ADSCs. The control and cocultured ADSCs were differentiated in the KoSR-containing the expression of neural maturation markers (Figs. 4 and 5). medium at the presence or absence of 10-8 M RA. Scale bar: 25 µm. Conclusion: Our findings are indicating that mouse ADSCs are capable of neural development in KoSR-containing media. Moreover, coculture with the ES cells efficiently improves neural differentiation of the ADSCs. Probably, secretion of cytokines, chemokines, interleukins and some growth factors by ES cells have a positive effect on the late maturation of ADSC-derived neurons. Non-contact coculture with the ES cells may be used as an efficient strategy to improve differentiation potential of adult stem cells for developmental studies and regenerative medicine. Figure 2- The expression of some neuron-specific genes in the freshly isolated stromal Reference: Bahmani L, Taha MF, Javeri A. Neural differentiation of adipose tissue- vascular fraction (SVF), third-passaged ADSCs (ADSC P3), brain tissue as the positive derived stem cells is improved following coculture with embryonic stem cells. control, and two-week differentiated ADSCs which were treated with different concentrations Neuroscience 2014; 272: 229-239. of RA.