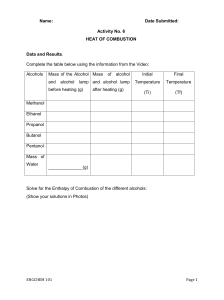
Combustion of Fuels CH105 Fall 2020 Experimental Objective: • Determine the heat of combustion in kJ/mol for a series of fuels. Learning Objectives: • Relate stoichiometry of reaction to heat produced • Reinforce concepts of enthalpy of formation, bond enthalpy, and Hess’ law, in a hands-on practical example • Determine the efficiency of combustion reactions under certain experimental conditions Big Picture Concepts: burning organic molecules for energy is a really big deal Background Reading: 5.1 & 5.3 of Chemistry 2e from OpenStax An alcohol is an organic compound that contains an oxygen – hydrogen bond also called an “OH” group. An example of an alcohol is methanol, CH3OH. Alcohols (like nearly all organic compounds) will react with oxygen to produce carbon dioxide and water. These reactions are called combustion reactions and are always exothermic. The energetic driving force for the reaction is the formation of the very strong carbon – oxygen and oxygen – hydrogen bonds. 2 CH3OH + 3 O2 → 2 CO2 + 4 H2O ∆H < 0 Sometimes when an alcohol or other organic molecule is burned the reaction does not go as written. For example if there is not sufficient oxygen present, the alcohol does not react completely and carbon monoxide or elemental carbon (called carbon black for its black color) can be formed. This means fewer carbon – oxygen and oxygen – hydrogen bonds are being formed. The net effect of this is that less energy will be produced. CH3OH + O2 → CO + 2 H2O 2 CH3OH + O2 → 2 C + 4 H2O Ethanol, CH3CH2OH, is an alcohol that contains two carbons and can be used as a fuel. In the USA, ethanol is typically produced from corn. Some cars run on an ethanol gasoline blended fuel called E85. At one time, ethanol was considered as a possible renewable fuel alternative to petroleum based gasoline. In the Midwest, E85 was seen as a double blessing, a fuel that is not dependent on crude oil, and also a fuel that would stimulate the agricultural economy of the area. In recent years, the use of ethanol as a fuel has dropped dramatically. While derived from corn or another plant, burning ethanol still produces carbon dioxide. A small amount of ethanol has been added to gasoline for several years to “oxygenate” the gasoline or to help promote more complete combustion. CH3CH2OH + 3 O2 → 2 CO2 + 3 H2O ∆H < 0 The purpose of this lab is to determine which alcohol would make a better fuel in terms of how much energy is produced per gram of alcohol burned. Though chemists typically give enthalpies of reaction in kJ/mol, for fuels kJ/g is more commonly used. This is done to make fuels with very different molecular weights more comparable. We will also look at the efficiency of combustion under our experimental conditions. Combustion of Fuels CH105 Fall 2020 To do before lab: 1. What is the specific heat capacity of water in J/g.°C? Give your source. 2. What is the specific heat capacity of Pyrex glass in J/g.°C? Give your source. 3. Use your textbook to find the enthalpies of formation for the following compounds in kJ/mol. Additionally, calculate the molecular weights of the three alcohols. A table would be a very good option here. water, H2O(l) carbon dioxide, CO2(g) methanol, CH3OH(l) ethanol , CH3CH2OH(l) propanol, CH3(CH2)2OH(l) Procedure Choose one of the alcohols, methanol, ethanol or propanol, and write a balanced combustion reaction for the reaction of one mole. Follow the procedure to measure the enthalpy of combustion for your alcohol. 1. Determine the mass of a clean, dry, 250 mL Erlenmeyer flask. (Try to use an Erlenmeyer flask that has previous charring on the bottom.) 2. Add approximately 100 mL of deionized water (record exact volume). 3. Clamp the neck of the Erlenmeyer to the ring stand at reasonable height so as to allow easy access for sliding the alcohol burning unit but also close enough to the burner to capture the heat from the combustion of the alcohol. 4. Weigh the unlit burner unit. Record the mass of the burner and record the name of the alcohol. Do not adjust the wick. 5. Record the starting temperature of the water. 6. Place the burner beneath your calorimeter and light the burner. Discard the extinguished match in a beaker of water. 7. Stir the water in the flask gently and slowly with the thermometer and heat until you measure a temperature approximately 20 ºC higher than the initial value. If your flame goes out, just relight and continue heating. 8. After the temperature has risen by 20 ºC, suffocate the flame by replacing the cap and stir the solution for about 30 seconds more to distribute the heat energy within the water. Once the temperature has stopped rising, record the value on the data sheet. 9. Remove the burner and reweigh it. 10. Once the flask has cooled to room temperature, empty the water and clean up your lab station. *Caution: Do not handle hot glassware with bare hands (silicone mitts are available), and do not place hot glassware directly on a cool bench surface. Combustion of Fuels CH105 Fall 2020 Analysis Determine the enthalpy of combustion in kJ/mol and kJ/g for each alcohol. Things to consider: What are you heating, just water or water and glass? What is the change in temperature? How much of the alcohol reacted? Use the enthalpies of formation that you looked up before lab to calculate the theoretical enthalpy of combustion for each alcohol. Compare the experimental enthalpies to the theoretical values. You can calculate the efficiency using the following calculation. 1 − |theoretical value − experimental value| x 100 % theoretical value Why is the process so inefficient? Cleanup Thoroughly wash and rinse all glassware. You will need to use soap and a scrub brush to wash the bottom of the flasks and remove as much of the charring as possible. Wipe down your work area. Don’t forget to wash your hands before you leave!