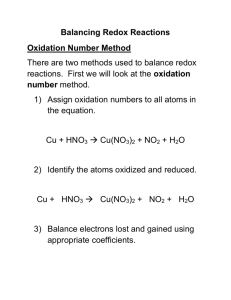
Balancing Redox Reactions Balancing Redox Reactions • We balance Redox reactions by making sure that the number of electrons lost by the reducing agent equals the number of electrons gained by the oxidizing agent. • Two methods are typically used the Oxidation Number Method and the Half-Reaction Method Oxidation Number Method 5? Step 1: Assign oxidation numbers to all atoms 0 +5 +1 -2 e +2 +5 -2 +4 -2 +1 -2 Cu + HNO3 → Cu(NO3)2 + NO2 + H2O Step 2: Identify the oxidized and reduced reactants and determine how many electrons each lost or gained Copper went from 0 to +2, therefore it was oxidized and lost 2 eNitrogen went from +5 to +4, therefore it was reduced and gained 1 e- Oxidation Number Method Step 3: Multiply one/both pairs by a factor which causes an equal number of electrons to be transferred Lost 2 e- Cu + HNO3 → Cu(NO3)2 + NO2 + H2O Gained 1 e- Therefore multiply by 2 Step 4: Complete balancing by inspection Cu + 2 HNO3 → Cu(NO3)2 + 2 NO2 + H2O Cu + 42 HNO3 → Cu(NO3)2 + 2 NO2 + 2 H2O Tracking electrons transferred per atom • You must ensure that you are tracking the number of electrons transferred per atom: Cu(s) + H2SO4(aq) → Cu2SO4(aq) + SO2(g) + H2O(l) Copper loses one electron per copper to form Cu(I) sulfate. Since there are two copper ions in each copper (I) sulfate, two copper atoms are required and 2 electrons total are transferred in the oxidation process. 2 Cu(s) + 2 H2SO4(aq) → Cu2SO4(aq) + SO2(g) + 2 H2O(l) Class/Homework • Complete questions 1-4 ONLY on the handout. • Read Textbook pp. 664-673 • Text Q’s p. 668, 671, 673.