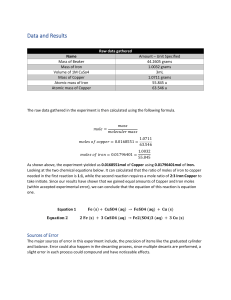
Javokhir Sadulloev 9/23/2021 Pre-Lab Outline for Lab 3 Abstract: To determine which reaction will likely occur using stoichiometry. Background: The terms needed for this lab will be defined below. Stoichiometry refers to the relationship between the quantities of reactants and products before, during, and following chemical reactions. Redox reactions are half reduction and half oxidation reactions. These changes can be evaluated in terms of lost of gained electrons. Elements electric negativity can determine whether they will lose or gain electrons. In case of Iron and Copper(II) Sulfate Solution iron is more reactive than copper and therefore will displace it in the reaction. This will be the main objective of this lab experiment.