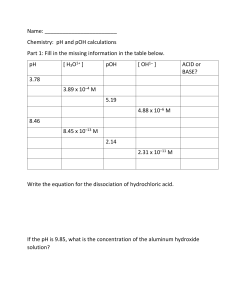
Chemistry Practice Name___________________ Formulas Kw = 1.00 x 10-14 pH = - log [H+] Kw = [H+] [OH-] pOH = - log [OH-] 1. Fill in the following table. [H+] pH 4.4 x 10-3 2.36 pH + pOH = 14 [H+] = 10-pH [OH-] = 10-pOH [OH-] 2.3*10^-12 pOH 11.64 acid or base acid 2.29*10^-10 9.64 4.3*10^-5 4.36 basic 4.55E-10 9.34 2.2 x 10-5 4.66 basic .0021 2.67 4.677E-12 11.33 acid 4.17E-10 9.38 2.40E-5 4.62 basic 1.47E-7 6.83 6.8 x 10-8 7.62 acid 1.23E-5 4.91 8.13E-10 9.09 acid 1.89E-4 3.8 based 5.3 x 10-11 10.2 4. What is the pH of a 4.8 x 10-5 M solution of hydrochloric acid? -log(4.8E-5 4.32 5. What is the pH of a 7.9 x 10-3 M solution of potassium hydroxide? -Log(7.9E-3 14-2.14 11.86 6. What is the concentration of a nitric acid solution that has a pH of 2.18? 10^-2.18=.0066M 7. What is the concentration of a sodium hydroxide solution with a pH of 12.54? 10^-12.54 2.88 8. List the six strong acids HBr H2SO4 HI HNO3 HCl HClO4 9. List four properties of a base and four properties of an acid. What color is BTB in acid, base, neutral solutions? Acids taste sour, turns the paper red, can corrode things, nuturalize bases. Bases turn blue natural doesn’t change the color 10. What is the concentration of a hydrochloric acid solution if 5.00 mL of the hydrochloric acid solution is exactly neutralized with 22.50 mL of a 0.100 M sodium hydroxide solution? 11. Complete the equations for the following: H2SO4 (aq) + KOH (aq) 🡪 Ca(OH)2 (aq) + HCl (aq) 🡪 Ba(OH)2 (aq) + H2C2O4 (aq) 🡪 12. Label the acid, base, conjugate acid and conjugate base in the following H2O + NH3 🡪 NH4+ + OH- HF + H2O 🡪 H3O+ + F- S2- + H2O 🡪 HS- + OH- 13. How many grams of sodium iodide are needed to make 500.0 mL of a 0.125 M aqueous solution of sodium iodide? 14. What is the concentration, in moles per liter, of a solution of 12.0 grams of sodium chloride in enough water to make 250.0 mL of the solution? 15. Describe the equipment and procedures for the titration lab and how to do the calculations. 16. How many times higher is the H+ concentration in a solution of pH of 2 than a solution with a pH of 6? 17. Calculate the concentration of hydrochloric acid (HCl) if 15.0 mL of HCl is just neutralized with 22.0 mL of 0.125 M sodium hydroxide, NaOH. 18. Calculate the concentration of sulfuric acid (H2SO4) if 15.0 mL of H 2SO4 is just neutralized with 22.0 mL of 0.125 M sodium hydroxide, NaOH. 19. Describe the H+/OH- concentrations for an acidic, basic and neutral solution.