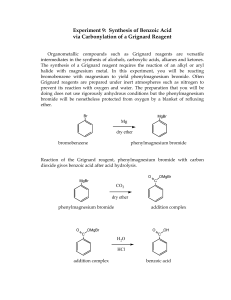
EXPERIMENT 3 The Grignard Reaction: Preparation of Benzoic Acid Introduction One of the most central topics of study in organic chemistry surrounds the discovery of new methods to prepare carbon–carbon bonds. The Grignard reaction is one of the most important classical methods for forming carbon–carbon bonds and was discovered in 1900 by Victor Grignard (who won the 1912 Nobel prize in chemistry for his discovery). Grignard reagents are organometallic compounds formed via the reaction between an alkyl or aryl halide with magnesium using diethyl ether as a solvent. The reactivity of Grignard reagents is defined by the carbon–magnesium bond that is formed in which the electronegativity difference (i.e., the more electronegative carbon inductively withdraws electron density from magnesium) leads to the formation of a partial negative charge on the carbon atom. As such, the carbon atom in a Grignard reagent acts as a good nucleophile and can react with a variety of electrophiles including aldehydes, ketones, acid halides, esters, and nitriles. Grignard reagents are most commonly reacted with carbonyl- or carboxyl-containing substrates to produce alcohol products. Aldehydes and ketones react with one equivalent of a Grignard reagent to produce a secondary and tertiary alcohol, respectively. Acid halides and esters react with two equivalents of a Grignard reagent to form a tertiary alcohol. The first addition of a Grignard reagent to a carboxyl functional group produces a ketone intermediate that is more reactive than the reactant and thus undergoes a second addition of a Grignard reagent to yield a tertiary alcohol. Grignard reagents are not only potent nucleophiles, but they are also very strong bases resulting from the stability of their conjugate acids (i.e., alkanes, aromatics). The reaction between a Grignard reagent and any proton sources (e.g., alcohols, amines, carboxylic acids, water) leads to the decomposition of these compounds. Further, Grignard reagents react with oxygen which also leads to their decomposition. As such, special preparations need to be taken to conduct Grignard reactions under conditions that exclude both water and oxygen. Chemical reagents needed for a Grignard reaction, especially the solvent (e.g., diethyl ether), need to be pre-dried to remove water before starting the reaction. Removal of water from the reaction equipment can be achieved by first rinsing all glassware with appropriate organic solvents and then heating the glassware in the presence of a water absorbent (e.g., calcium chloride). Further protection against water and oxygen is provided by a volatile organic solvent (e.g., diethyl ether) as vapours of the solvent provide an insulating blanket around the reaction mixture. Thus, if great care is taken while preparing and running a Grignard reaction, then high yields of the desired product can be obtained. Objective In this experiment, you will prepare phenylmagnesium bromide and subsequently react this Grignard reagent with solid carbon dioxide to form benzoic acid. You will identify your product using IR and NMR spectroscopy. References D. Klein, Organic Chemistry: 4th Edition, John Wiley and Sons, Hoboken, 2021, pp. 529–584 (Chapter 12) Pre-lab Preparation Besides preparing your lab report as outlined in the Laboratory Information section (be sure to doublecheck the requirements for synthetic reactions), familiarize yourself with distillation, temperature control of reactions (see the Laboratory Techniques section), NMR spectroscopy (see the Spectroscopy section) and the safety information for the chemicals that will be used in this experiment. 1. 2. 3. 4. 5. Watch the pre-lab video on A2L. Prepare your lab report as outlined in the Laboratory Information section: a. Title, Objective, and the Full Written Procedure are required to be allowed entry into the labs Determine the theoretical percent yield for your experiment along with the molecular weight of your reagent and product. Draw and fill out the flowchart below in the pre-lab section of your report. Complete and submit the Pre-Lab quiz on Avenue2Learn Table 3-1. Safety information for the chemicals employed in Experiment 3. COMPOUND HAZARD STATEMENTS Bromobenzene Harmful if swallowed Causes skin or eye irritation Flammable liquid Toxic to aquatic life Calcium chloride Harmful if swallowed Causes skin or eye irritation Diethyl ether Harmful if swallowed or inhaled May cause drowsiness or dizziness Extremely flammable liquid and vapours Dry ice (solid carbon dioxide) Causes skin burns or frostbite May cause drowsiness or dizziness Hydrochloric acid Harmful if swallowed Causes serious skin burns or eye damage Iodine Harmful if swallowed or inhaled Causes skin or eye irritation May cause damage to organs through prolonged or repeated exposure if inhaled Toxic to aquatic life Magnesium Flammable solid Contact with water releases a flammable gas Grignard reagent solution Harmful if swallowed Causes serious skin burns or eye damage Highly flammable liquid and vapours Reacts violently with water Sodium hydroxide Harmful if swallowed Causes serious skin burns or eye damage Experimental Procedure Part A. Preparation of Phenylmagnesium Bromide (Grignard Reagent) Equipment Note: Grignard reagents are highly water-sensitive and all glassware must be properly DRIED prior to use! 1. If needed, first clean your glassware with soap and water. Make sure to dry all of the glassware required for this reaction including a 10 mL cylindrical vial, a water condenser, a magnetic stir bar, a blue connector, a drying tube, and a graduated cylinder with a sequence of first rinsing with acetone (to remove traces of water) and then rinsing with dry ether (to remove traces of acetone). Reagent Note: Two types of diethyl ether will be used for this experiment. Dry ether is to be used for rinsing glassware and liquid-liquid extraction (large bottle with dispenser). Anhydrous ether is only to be used as a solvent for your reaction (small metal bottle). 2. Prepare a drying tube by placing a small piece of glass wool at the end of a drying tube, fill the tube with calcium chloride, and cap the tube with another small piece of glass wool. 3. Set up a reflux apparatus using a 10 mL cylindrical vial and a water condenser fitted with the calcium chloride drying tube. (Note: do not turn on the water for the condenser as it is not needed for the first step of this procedure.) Reaction apparatus for the preparation of phenylmagnesium bromide (Part A). 4. Prepare a communal ice bath for each lab bench in case any reactions become too vigorous. 5. Add a magnetic stir bar, 0.17 g Mg turnings, a single crystal of iodine to the vial, and then IMMEDIATELY reassemble the reflux apparatus. 6. To dry your reaction setup, gently heat the vial on a hotplate using an aluminum heating block and magnetic stirring without cooling water. Continue to heat your vial until a light purple vapour is generated. Remove the vial from heat and cool to room temperature. Equipment Note: DO NOT disassemble your reflux apparatus until it has completely cooled to ensure that water is not introduced into your reaction! 7. Remove the vial from the condenser and directly place a cap on the vial. Add 3 mL of anhydrous dry ether and 0.75 mL (1.1 g) of bromobenzene to the vial and place the cap back on the vial. Immediately reassemble the reflux apparatus, begin magnetically stirring the reaction mixture, and turn on the water to start a slow flow of water through the condenser. 8. If you have performed these steps carefully, the reaction should begin immediately or after slightly warming the mixture with your hand against the bottom of the flask (the stirring action of the magnetic stirrer also helps). You will recognize that the reaction has begun by the cloudy appearance and eventual boiling of your ether layer. Procedure Note: If the reaction does not start, the following steps should be carried out: Using a dry stirring rod, crush a piece of magnesium firmly against the bottom of the vial, taking care not to break the vial. f the reaction does not start, gently warm the vial while stirring. Remove from heat and check if your reaction continues to boil. If not, try step 1 again or move on to step 3. If the reaction still doesn’t start, add a couple of drops of 1,2-dibromoethane. Stir for a couple of minutes. If there is still no reaction, you will have to restart your experiment. Ensure that all of your glassware is clean and dried using a rinse sequence of water, acetone, and dry ether. 9. Once the reaction has started, add an additional 2 mL of anhydrous dry ether through the top of the condenser using a glass pipette. The reaction mixture should continue to boil, but if it becomes sluggish, gently warm the vial. Continue to stir the reaction mixture for 30 minutes. If at any point during this step the volume of ether decreases, add a few more millilitres of anhydrous dry ether to keep the vial about half full. You have now prepared a solution of phenylmagnesium bromide in ether and it should be used immediately in the next step. Part B. Reaction of Phenylmagnesium Bromide with Carbon Dioxide 10. Only once your phenylmagnesium bromide solution is prepared, obtain 4.0 g of solid carbon dioxide in a beaker. 11. With vigorous stirring using a glass rod, slowly add the prepared solution of phenylmagnesium bromide into the beaker containing solid carbon dioxide. Stir the mixture until it becomes a sticky solid. 12. Once the solid carbon dioxide has disappeared, slowly add a mixture of water (7 mL), ice (7 g), and concentrated hydrochloric acid (1.0 mL) and mix thoroughly. Make sure that any remaining magnesium has been completely dissolved before moving onto the extraction step. Equipment Note: Take a moment to empty your drying tube into the Solid Waste container. If you wait, calcium chloride will absorb too much water and will be very difficult to remove from your tube. Procedure Note: Liquid-liquid extractions are challenging! Be sure to review this technique prior to coming to lab. It is most important in extractions that you are able to correctly identify the organic (i.e., ether) and aqueous layers. To ensure that mistakes are not made, all collected layers should be kept until the end of your experiment! 13. Add 5–7 mL of dry ether to your beaker. Pour this liquid mixture into a separatory funnel. The upper layer is the ether phase and the bottom layer is the aqueous phase. Separate both layers into different beakers. If needed, label your beakers so that you do not confuse the phases. 14. In order to obtain all of your product from the water layer, put the aqueous phase back into the separatory funnel and extract it with 5 mL of dry ether. Combine this second portion of ether with the first ether portion removed from the funnel. 15. In order to purify your product, put the ether phase back into the separatory funnel and extract it THREE times with 4 mL of 1.0 M NaOH. Remove the aqueous (bottom) layer each time and leave the ether (upper) layer in the separatory funnel. The aqueous layers can be combined together after each extraction. Ensure that your aqueous phase does not contain any ether. 16. In order to precipitate your product from the water layer, acidify the aqueous phase with concentrated hydrochloric acid to pH 3. Use pH paper to check the pH of the solution. If precipitate does not form, try to cool the mixture in an ice bath. 17. Collect the product by vacuum filtration using a Hirsch funnel. Wash the product with two portions (~2 mL) of water. Dry the product by drawing air through the funnel for a few minutes. Record the weight of the product. For your Report 1. Determine the product yield. 2. Obtain an IR spectrum of your product and assign the most important signals. 3. Assign the 1H and 13C NMR spectra of your product. Equipment Note: Your microscale kit must be COMPLETELY CLEAN when returned to your TA. First clean and scrub your glassware with soap and water and then make a final rinse with acetone. If your glassware is still not clean, you may have to rinse them with an acid or base wash.