Uploaded by
jroque10
Nuclear Physics Presentation: Atomic Structure & Quantum Theory

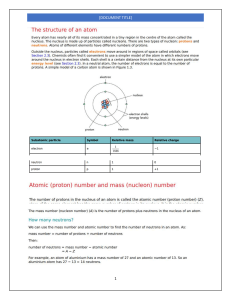
Nuclear Physics Atomic Structure Bohr Model Protons (atomic # Z) • Positively charged • Located in the nucleus – Mass = 1.6726 x 10 -27 kg • All atoms of an element have the same number of protons, whether they are radioactive or not. • Dictates position on periodic table and chemical identity • Protons and Neutron, and their associated forces, affect an atom’s radioactive properties Neutrons • No charge • Located in the nucleus • 1800 times as heavy as an electron. – Mass= 1.6749 x 10 -27 kg • Neutron + proton = Nucleon Electrons • Negatively charged – Neg. charge of 1.6022 x 10-19 Coulomb – Mass = 9.109 x 10-31 kg • Orbit around the nucleus • Determine atom’s chemical properties • Less mass • Higher energy? Electron Shell Configuration Sl.No Electron Shell Maximum Capacity 1 K Shell 2 electrons 2 L Shell 8 electrons 3 M shell 18 electrons 4 N shell 32 electrons ELECTRON VOLT • Base unit of energy • Amount of energy acquired by an electron when accelerated through an electrical potential of 1V. Atomic Shorthand X = Chemical Symbol A= Atomic mass Z= Number of protons Subtract A-Z to find the number of neutrons. Chemical Shorthand • X= elemental atom A Z X • Z= Atomic Number • # of protons • N= Neutron Number N • A= Mass Number • Z+N • Total number of nucleons Mass Unit • Universal mass unit: equal to ½ the mass of a Carbon atom 1.66054 x 10 -27 kg • Atomic Mass Unit (AMU) 1/12 mass of stable Carbon-12 atom One AMU is about the same as one proton, and also about the same as one neutron. One AMU is 1.66x10-27 kg Vocabulary words • Valent Electrons: outer electrons that interact to form molecules • Valence: is an atom’s ability to lose or gain an e- so that it can achieve a stable e- configuration. Usually determined by the number of e- in outermost shell (valence shell) – Octet Rule: Most stable form of atom. Main-group elements tend to undergo reactions that leave them with 8 outer shell e-. They react in such a way that they attain an e- configuration like an inert gas in which the s and p orbitals are filled in their valence e- shell (ns2np6) Vocabulary words • Atom: smallest part of an element with the same chemical properties. Contains 3 types of elementary particles: p+, n, e• Element: An element is a fundamental substance that cannot be chemically broken down any further. • Molecules :are formed from combinations of elements • Matter: Molecules make up matter 2 E = mc E= energy M= mass C= speed of light (3 x 108m/sec) Matter and Energy are directly related, matter can be turned into energy and energy can be turned into matter. Quantum Theory • Subatomic particles-tiniest things we know • Collaborative effort by early 20th century physicists: Niels Bohr, Erwin Schrodinger, Wolfgang Pauli, Max Born, Max Planck, Werner Hiesenberg • Explains the nature and behavior of matter and energy on the atomic and subatomic levels. Quantum theory • the study of the jumps from one energy level to another as it relates to the structure and behavior of atoms. May the Forces be with You • Nature consists of mainly matter and the forces that govern the behavior of matter • Force is a term related to interaction between different parts of matter – Gravitational – Electromagnetic – Weak – Strong The Forces • Gravitational: result of the mass of matter (holds the solar system together, helps create centrifugal force) • Electromagnetic: dominate role in holding atoms together and interactions between atoms, molecules, biomolecules, etc • Strong: hold a nucleus together (p-p, n-n, p-n) • Weak: nuclear transformation Gravitational • Best described in Einstein’s Theory of Relativity • force proportional to masses of interacting bodies and inversely proportional to the square of the distance between them • Newton's law of universal gravitation simplifies to F = mg, where m is the mass of the body and g is a constant vector with an average magnitude of 9.81 m/s2. The acceleration due to gravity is equal to g. Gravitational • every object in the universe exerts a gravitational force on everything else. • The effects of gravity depend on two things: the mass of two bodies and the distance between them. • is about 10-36 times the strength of the strong force Electromagnetism • has both attractive and repulsive properties that can combine or cancel each other out • two charges: positive and negative • keeps atoms together: the positively charged nucleus and the negatively charged electrons attract each other • messenger particle of electromagnetism is the photon Magnetism • A magnetic field describes a volume of space where there is a change in energy. – a magnetic field is produced whenever an electrical charge is in motion. – The spinning and orbiting of the nucleus of an atom produces a magnetic field as does electrical current flowing through a wire. – The direction of the spin and orbit determine the direction of the magnetic field. – motion of an electric charge producing a magnetic field is an essential concept in understanding magnetism Magnetism • All the electrons produce a magnetic field as they spin and orbit the nucleus • The direction of spin and orbit of the electron determines the direction of the magnetic field. • When two electrons are paired, they spin and orbit in opposite directions. Since the magnetic fields produced by the motion of the electrons are in opposite directions, they add up to zero. • The overall magnetic field strength of atoms with all paired electrons is zero. Coulombs Law • Simplified: like charges repel, opposites attract. K = Coulombs law constant Q =quantity of charge on objects 1 and 2 D= distance F= force Strong Force • range is limited to subatomic distances • Also called nuclear force • keeps quarks together inside protons and neutrons • keeps protons and neutrons inside atomic nuclei (binds nucleons). • messenger particle is the massless gluon, so named because it "glues" elementary particles together Weak Force • only on the extremely short distance scales found in an atomic nucleus • stronger than electromagnetism, but its messenger particles (W and Z bosons) are so massive that they do not faithfully transmit its intrinsic strength. • responsible for radioactive decay Force Particle Mass force Gravitational Force Graviton 0 weak Electromagnetic Force Photon 0 weak Weak Nuclear Force Weak Gauge Bosons 86, 97 strong Strong Nuclear Force Gluon 0 strong THE UNIFICATION OF FORCES: Electromagnetic and gravitational forces get weaker as you get further away while strong and weak get stronger. Bosons are the particles which transmit the different forces between the matter particles Quantum Theory • Stability of an atom is governed by nuclear (strong and weak) and electromagnetic forces • Strong is attractive • Electromagnetic is repulsive • Balance of these forces (attraction and repulsion) must be maintained for stability • Unbalanced = radioactive Quantum Theory • Wave particle duality • changes in electric and magnetic fields are always coupled, oscillating • relationship between frequency and wavelength • distance per cycle × cycles per second = distance per second = c • The speed of electromagnetic radiation was computed to be around 3×108 m/s • Photons travel the same as light • All photons travel at the speed of light. solve for wavelength or frequency Electromagnetic Spectrum Plank’s Constant • Max Plank: one of the founder’s of the quantum theory, energy is not continuous • 1900- discovered the physical constant reflecting the sizes of energy quanta in quantum mechanics • 6.626×10-34 J/Hz • The Proportionality constant between energy (E) of a photon and the frequency (v) of its associated electromagnetic wave. (relation between energy and frequency) C= 3 x 10 8 m/s Electromagnetic Radiation • form of energy emitted and absorbed by charged particles • wave-like behavior as it travels through space. • Photons-massless energy • Gamma = higher frequency, shorter wavelength, and higher energy • Nuclear Binding Energy: the direct measurement of energy it takes to hold the nucleons together and is determined when the loss of mass is converted into energy released during a nuclear reaction • The energy holding the nucleus together. Some of the mass of the nucleus is converted to energy to hold it together and that is why the nucleus weighs less than if you would add all the p+ and n together in the nucleus ΔE = Δmc2 • Typical Binding Energy value is 6-9 MeV – Amt of energy supplied to remove a single nucleon from the nucleus • The greater the binding energy, the more stable the nucleus Electron Binding Energy The energy needed to completely remove an electron from a shell of an atom • Relative to the given shell • Increases with the Z number…Why? • Where is the binding energy the greatest? Electron Binding Energy: • Energy needed to move an electron to one shell from another is equal to the difference in binding energy. • Greatest amount of energy at the inner most shells (K shell) Mass Deficit • When a proton and neutron fuse together, their new mass is less than the sum of the individual parts. • Mass deficit is the phenomenon wherein nucleons, the protons and neutrons that are being fused together to make up an atomic nucleus, each give up a little mass. • This is converted into binding energy Quantum Theory •Any imbalance of the forces governing an atom can lead to ionization as it seeks stability. –What do you think will happen if energy is imparted on an atom? •What are some things an atom might do to try to regain stability? Quantum Theory •What do you think will happen if a charged particle interacts with an atom? –What forces become unbalanced? –What does the atom need to do to regain stability? For Next week… •Complete handout •Review conversions •Study for Quiz