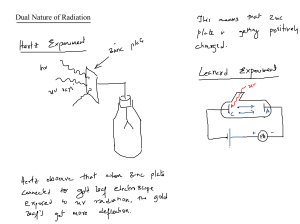
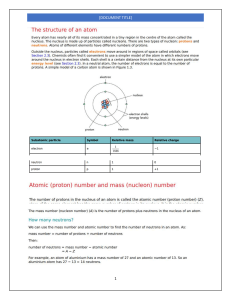
NMT 1613 Nuclear Physics Historical overview • 1895- Wilhelm Roentgen discovers X-Rays • 1896-Henri Becquerel discovers radioactivity • 1889- Marie Curie discovers Radium • 1913- Georg de Hevesy-developed the tracer approach • 1927- first human study Historical Overview • 1930’s-invention of a cyclotron • Post WWII- nuclear reactors were used for medical radioisotope production • 1951-1st Rectilinear Scanner • 1958- Anger Camera (Paul Anger) • 1960’s-most studies used I-131 • 1964- 99m Tc Nuclear Medicine Today • Development of technology and applied mathematics = SPECT and PET development • PET imaging and Fusion imaging • Over 100 different procedures • Any organ or organ system, psychology, research • Radiotherapy/radioimmunotherapy Quantities vs Units • Quantity describes WHAT is measured whereas a Unit describes HOW much. – Quantity: physical properties and processes eg. Time and Energy Fundamental: stands alone (eg time, distance, mass) Derived: combinations of fundamental quantities (eg. Energy is k*m2/ sec2) Scalars: • Scalar Quantity: a simple physical quantity that is not changed by coordinate system rotations or translations (in Newtonian mechanics) Example: driving 85 mph Vectors • Vectors: have a measurable amount in a distinct direction – quantities that have both magnitude and direction Eg. Driving 85 mph west English Vs. Metric English system: inches, foot, pounds, seconds Metric System: meters, grams (kilograms), seconds Basic Conversions • Distance: 1 inch = 2.5 centimeters 1 foot = .3048 meters (30 cm) How many centimeters is someone who is 5’7”? Basic Conversions • Weight 1 pound = 16 ounces 1 kilogram = 1,000 grams 1 kilogram= 2.2 pounds Convert 140.5 lbs into kilograms: In terms of Radiation Quantity: energy SI unit: Joule (J) Traditional Unit: electron volts (eV) Conversion: 1 Joule = 6.242 x1018 electron volts 1.602 x 10-19 J = 1 eV What is the energy of 99mTc in Joules? How about In-111? In terms of Radiation….. Quantity: radioactivity SI unit: Becquerel Traditional Unit: Curie Conversion: 1 Bq = 2.703 x 10-11 curies 3.7 x 1010 Bq = 1 Ci 37 MBq = 1 mCi 1 Ci = 1000 mCi Becquerel: the activity of a quantity of radioactive material in which one nucleus decays per second. (SI unit) radioactivity(in Bq) = Curie: (traditional unit) roughly the activity of 1 gram of the radium isotope 226Ra 1 Ci = 3.7 × 1010 decays per second A patient arrives for a bone scan. The dose from the radiopharmacy is labeled as 925 MBq but the charting system requires traditional units . How many Curies is this? How many mCi? 37 MBq = 1 mCi In terms of Radiation: Practical units of Measure Quantity: Absorbed Dose-amount of energy deposited as radiation interacts with matter SI units: Grays Traditional Units: rad (radiation absorbed dose) Conversion: 1 gray (Gy) = 100 rads 1x 10-2 Gy = 1 rad Gray: amount of radiation required to deposit 1 joule of energy in 1 kilogram of any kind of matter (J/kg) rad: corresponding traditional unit. 0.01 J deposited per kg. 100 rad = 1 Gy. Conversion: 1 gray (Gy) = 100 rads 1x 10-2 Gy = 1 rad You know that an average absorbed dose to the bladder for a patient receiving 10 mCi 99m TcMag3 is 263 rads. How much of the dose is absorbed when measures in Gy’s? In Terms of Radiation: Practical units of Measure Quantity: Dose Equivalent - Equal doses of radiation result in different amounts of damage to living tissue - Measure of biologic effect of radiation - Product of the absorbed dose in rads and weighting factors. Sievert (Sv)- more of a weighted average of the absorbed dose, designed to represent the health effects of exposure. (SI unit) also measured in J/kg Traditional Unit: Roentgen Equivalent Man (rem) Conversion: 1 Sv = 100 rems 1 x 10-2 Sv = 1 rem There has been a radioactive spill in your department in which a patient was unnecessarily exposed to radiation and now you have to determine the amount of radiation equivalent to that patient receiving a dose. The dose equivalent of 253 rems has been determined. What is the dose equivalent in Sv’s? In Terms of Radiation: Fundamental units of Measure Quantity: Exposure SI unit: Coulomb/kg of air (C/kg air) TI unit: Roentgen (R) Conversion: 1 C/kg air = 3876 Roentgen 2.58 x 10-4 C/kg air = 1 R Coulombs Law: describes interaction between electrically charged particles. Coulomb: unit of electrical charge, charge transported by a steady current of one ampere/sec. Coulomb in kg of air = the amount of radiation required to create 1 coulomb of charge of each polarity in 1 kilogram of matter. - SI unit, measured in C/kg Roentgen - traditional unit - the amount of radiation required to liberate 1 esu of charge of each polarity in 1 cubic centimeter of dry air. Conversion: 1 C/kg air = 3876 Roentgen 2.58 x 10-4 C/kg air = 1 R Atomic Review Atomic Structure Bohr Model Protons (atomic # Z) •Positively charged •Located in the nucleus –Mass = 1.6726 x 10 -27 kg •All atoms of an element have the same number of protons, whether they are radioactive or not. •Dictates position on periodic table and chemical identity •Protons and Neutron, and their associated forces, affect an atom’s radioactive properties Neutrons •No charge •Located in the nucleus •1800 times as heavy as an electron. –Mass= 1.6749 x 10 -27 kg •Neutron + proton = Nucleon Electrons •Negatively charged –Neg. charge of 1.6022 x 10-19 Coulomb –Mass = 9.109 x 10-31 kg •Orbit around the nucleus •Determine atom’s chemical properties •Less mass •Higher energy? Electron Shell Configuration Sl.No Electron Shell Maximum Capacity 1 K Shell 2 electrons 2 L Shell 8 electrons 3 M shell 18 electrons 4 N shell 32 electrons ELECTRON VOLT •Base unit of energy •Amount of energy acquired by an electron when accelerated through an electrical potential of 1V. Chemical Shorthand • X= elemental atom X • Z= Atomic Number •# of protons • N= Neutron Number • A= Mass Number •Z + N •Total number of nucleons Atomic Shorthand X = Chemical Symbol A= Atomic mass Z= Number of protons Subtract A-Z to find the number of neutrons. Mass Unit •Universal mass unit: equal to ½ the mass of a Carbon atom 1.66054 x 10 -27 kg •Atomic Mass Unit (AMU) 1/12 mass of stable Carbon-12 atom One AMU is about the same as one proton, and also about the same as one neutron. One AMU is 1.66x10-27 kg Vocabulary words • Valent Electrons: outer electrons that interact to form molecules • Valence: is an atom’s ability to lose or gain an e- so that it can achieve a stable e- configuration. Usually determined by the number of e- in outermost shell (valence shell) –Octet Rule: Most stable form of atom. Main-group elements tend to undergo reactions that leave them with 8 outer shell e-. They react in such a way that they attain an e- configuration like an inert gas in which the s and p orbitals are filled in their valence e- shell (ns2np6) Vocabulary words •Atom: smallest part of an element with the same chemical properties. Contains 3 types of elementary particles: p+, n, e•Element: An element is a fundamental substance that cannot be chemically broken down any further. •Molecules :are formed from combinations of elements •Matter: Molecules make up matter For Next Week: • Read chapters 1 and 2 in your Physics book • Read Math Book pages 26-39 • Homework handout • Log onto Canvas for this course.