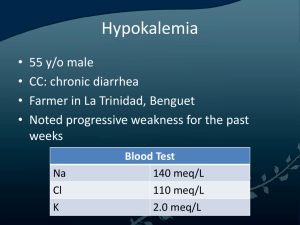
APPENDIX B-2 CLINICAL PROTOCOL Management of pediatric patients with Hypokalemia TITLE: IDENTIFICATION NUMBER: CPRO 23043 HOSPITAL(S): All HMC Hospitals with Pediatric facilities (HGH, Al Wakrah, Al Khor, Cuban hospital) ORIGINAL DATE: October 2023 LAST REVISION DATE: October 2023 NEXT REVIEW DATE: October 2026 Sheet No. 1 of 11 1.0 PURPOSE (AIM): 1.1 To provide Pediatric physicians and nurses the essential guidelines for management of patients with Hypokalemia 2.0 DEFINITIONS: 2.1 Hypokalemia is defined as serum potassium level of less than 3.5 mEq/L, and is further divided into: Severe hypokalemia – Potassium level less than 2.5 mEq/L Moderate hypokalemia – Potassium level between 2.5 and 3 mEq/L Mild hypokalemia – Potassium level between 3 and 3.5 mEq/L 2.2 For potassium levels, mEq/L and mmol/L are used interchangeably throughout the guideline as they denote the same value. 3.0 APPLIES TO: 3.1 All physicians, nurses, and pharmacists 4.0 PATIENT GROUP: 4.1 0 to less than 14 years of age 5.0 EXCEPTIONS: 5.1 NICU patient currently admitted in NICU 6.0 TARGET AREAS: 6.1 6.2 6.3 6.4 Pediatric Emergency Centers Pediatric Inpatients Pediatric Intensive Care Units Outpatient Departments 7.0 GUIDELINES: Governance, Leadership & Direction Corporate Clinical Practice Guidelines Committee APPENDIX B-2 CLINICAL PROTOCOL Management of pediatric patients with Hypokalemia TITLE: IDENTIFICATION NUMBER: CPRO 23043 HOSPITAL(S): All HMC Hospitals with Pediatric facilities (HGH, Al Wakrah, Al Khor, Cuban hospital) ORIGINAL DATE: October 2023 LAST REVISION DATE: October 2023 NEXT REVIEW DATE: October 2026 Sheet No. 2 of 11 7.1 Causes of Hypokalemia 7.1.1 Pediatric hypokalemia is due to one or a combination of the following mechanism 7.1.1.1 Excessive loss of potassium via the gastrointestinal tract(most common cause), kidney, or skin 7.1.1.2 Decreased potassium intake 7.1.1.3 Increased intracellular movement of potassium 7.2 Clinical Manifestation 7.2.1 Clinical manifestations vary depending on the severity and acuity of hypokalemia. 7.2.2 Symptoms do not generally manifest until serum potassium is below 3mEq/L except when there is a rapid fall in serum potassium 7.2.3 Ascending skeletal muscle weakness manifesting as hypotonia, hyporeflexia and/or fasciculations are seen with potassium levels less than 2 mEq/L . This may progress to paralysis of skeletal muscles, including respiratory muscles, leading to respiratory failure and death . 7.2.4 Smooth muscle weakness in the form of ileus manifesting as abdominal distension, anorexia, nausea, vomiting, and/or constipation. 7.2.5 Renal manifestations: Prolonged hypokalemia can cause renal dysfunction, particularly impaired concentrating ability that presents as polyuria and/or polydipsia. 7.2.6 Cardiac arrhythmias: premature atrial and ventricular complex beats,sinus bradycardia, paroxysmal atrial or junctional tachycardia, atrioventricular block, and ventricular tachycardia or fibrillation. 7.3 Assessment (Appendix- 1) 7.3.1 7.3.2 7.3.3 7.3.4 Because severe hypokalemia is a potentially life-threatening condition, initial management takes precedence over any diagnostic evaluation. In mild hypokalemia with a history of present illness that suggests a clear etiology such as viral Gastrointestinal illness or medications like salbutamol, insulin, amphotericin or diuretic therapy: no need for further work up. Patients whose etiology is unclear based on their history and clinical presentation require further diagnostic workup. These include patients with recurrent hypokalemia, hypertension, history of familial periodic paralysis, recurrent chest infections, and those with underlying renal or endocrine disorder Blood Test 7.3.4.1 Governance, Leadership & Direction Complete blood count, Serum electrolyte including Blood sugar and Renal Corporate Clinical Practice Guidelines Committee APPENDIX B-2 CLINICAL PROTOCOL Management of pediatric patients with Hypokalemia TITLE: IDENTIFICATION NUMBER: CPRO 23043 HOSPITAL(S): All HMC Hospitals with Pediatric facilities (HGH, Al Wakrah, Al Khor, Cuban hospital) 7.3.4.2 7.3.5 ORIGINAL DATE: October 2023 LAST REVISION DATE: October 2023 NEXT REVIEW DATE: October 2026 Sheet No. 3 of 11 function test, Magnesium, Phosphorus and Blood Gas Spot urine sodium, potassium, chloride, creatinine, Renin Aldosterone (if suspected Endocrine/ Renal etiology) Electrocardiography (ECG) 7.3.5.1 Perform ECG if signs/symptoms of hypokalemia, risk ofcardiac arrhythmia, or serum potassium <3 mmol/L. 7.3.5.2 Flat or inverted T waves; ST depression. 7.3.5.2.1 Less commonly: QT Long, wide QRS, U wave. 7.3.5.2.2 With digitalis toxicity: all of the above plus various AV blocks, bradycardias, and arrhythmias of any type. 7.4 Treatment/Management (Appendix- 2) 7.4.1 The urgency of therapy depends upon the severity of hypokalemia, and the rate of decline in serum potassium concentration. 7.4.2 The primary aim in hypokalemia management is to correct dangerously low potassium concentrations (e.g. < 2.5 mEq/L) to safe levels (>3.0mEq/L), Serum concentration Severity Recommended 1st line replacement 3.0-3.5 mEq/L Mild 2.5-3.0 mEq/L Moderate <2.5 mEq/L Severe Enteral Asymptomatic – Enteral Symptomatic patient- consider IV Enteral replacement not feasible- IV IV (If chronic, oral may be used upon nephrology advise) 7.4.3 The amount of replacement therapy depends on the 7.4.3.1 Cause of the hypokalemia Governance, Leadership & Direction Corporate Clinical Practice Guidelines Committee APPENDIX B-2 CLINICAL PROTOCOL Management of pediatric patients with Hypokalemia TITLE: IDENTIFICATION NUMBER: CPRO 23043 HOSPITAL(S): All HMC Hospitals with Pediatric facilities (HGH, Al Wakrah, Al Khor, Cuban hospital) ORIGINAL DATE: October 2023 LAST REVISION DATE: October 2023 NEXT REVIEW DATE: October 2026 Sheet No. 4 of 11 7.4.3.2 Presence of any acid-base disorder 7.4.3.3 Ongoing excessive losses. 7.4.4 Treat underlying cause when identified, Magnesium supplementation — Hypomagnesemia may accompany hypokalemia. Magnesium supplementation may need to be considered, especially with difficulty correcting hypokalemia. 7.4.5 Oral potassium therapy is safest and should be used whenever possible. Inj. Potassium Chloride (KCL) 40 mEq/L is generally safe at usual IV fluid rates 7.4.6 When IV Potassium chloride correction is used, the upper limit for potassium administration rate is 0.2mEq/kg/hr in the pediatric ward setting and 0.5mEq/kg/hr in the ICU setting. Administering at 0.5mEq/kg/hr requires a central line and needs frequent serum potassium monitoring and ECG monitoring. 7.4.7 Potassium shall be given IV infusion only 7.4.8 In acute hypokalemia, stop potassium replacement when serum potassium is ≥3.5 mEq/L (except that which is being given as part of IV fluids) 7.4.9 Chronic hypokalemia: 7.4.9.1 Chronic hypokalemia at lower levels tend to be better tolerated by the patient and are less likely to require urgent interventions 7.4.9.2 In children with asymptomatic chronic hypokalemia, potassium supplementation may be required indefinitely, especially if the underlying cause cannot be corrected (for example, Type I or II Renal tubular acidosis). 7.4.9.3 Potassium-sparing diuretics — Potassium supplementation by itself is less effective in tubulopathies such as Bartter or Gitelman syndromes, where there is ongoing renal wasting of potassium. Use of a potassium- sparing diuretic such as amiloride may attenuate these losses. 7.4.9.4 Children with hyperaldosteronism may benefit from spironolactone to reduce the urinary potassium effect of aldosterone. Governance, Leadership & Direction Corporate Clinical Practice Guidelines Committee APPENDIX B-2 CLINICAL PROTOCOL Management of pediatric patients with Hypokalemia TITLE: IDENTIFICATION NUMBER: CPRO 23043 HOSPITAL(S): All HMC Hospitals with Pediatric facilities (HGH, Al Wakrah, Al Khor, Cuban hospital) ORIGINAL DATE: October 2023 LAST REVISION DATE: October 2023 NEXT REVIEW DATE: October 2026 Sheet No. 5 of 11 7.4.10 Oral potassium therapy: 7.4.10.1 Dose: Acute replacement dose 1 - 2 mmol/kg/dose orally (maximum 20 mmol per dose) Maintenancedose 2 - 5 mmol/kg/DAY orally in divided doses (maximum 20 mmol perdose) Dose may be repeated, after checking serum potassium level, toa maximum of 5 mmol/kg/DAY (maximum daily dose 50 mmol) 7.4.10.2 Medication forms: Potassium chloride (KCL) Rapid absorption Preferred in patients with concomitant hypochloremia or metabolic alkalosis Potassium phosphate Often used in proximal tubular dysfunction since there is loss of bothpotassium and phosphorus, may be used in children with diabetic ketoacidosis with symptomatic phosphatemia Potassium acetate Commonly used in DKA, allowing for correction of hypokalemia as well as acidosis Potassium citrate Generally used in children with hypokalemia and metabolic acidosis, e.g., renal tubular acidosis 7.4.10.3 Adverse Effects: 7.4.10.3.1 Gastrointestinal: nausea, flatulence, vomiting, abdominal pains, diarrhea or bleeding 7.4.10.3.2 Poor adherence is a major limitation to use. Smaller divided doses taken with or after food may minimize gastric irritation. 7.4.11 Intravenous dosing (Refer to Appendix 2) 7.4.11.1 Potassium must be diluted prior to parenteral administration 7.4.11.1 Rapid intravenous administration or overdose may cause cardiac arrest 7.4.11.2 Administer via an infusion pump 7.4.11.3 Concentration of Potassium chloride stock solution: 2 mmol/ml 7.4.11.4 Potassium Dosing and Rate of Infusion 7.4.11.4.1 For peripheral lines, potassium concentrations should be 20 – 40 mmol/L. Only in exceptional circumstances, up to 60 mmol/L can be given via peripheral lines Governance, Leadership & Direction Corporate Clinical Practice Guidelines Committee APPENDIX B-2 CLINICAL PROTOCOL Management of pediatric patients with Hypokalemia TITLE: IDENTIFICATION NUMBER: CPRO 23043 HOSPITAL(S): All HMC Hospitals with Pediatric facilities (HGH, Al Wakrah, Al Khor, Cuban hospital) ORIGINAL DATE: October 2023 LAST REVISION DATE: October 2023 NEXT REVIEW DATE: October 2026 Sheet No. 6 of 11 7.4.11.4.2 In the Pediatric floor, a maximum concentration of 40 mmol/L in IV fluids can be given after approval by the Pediatric Consultant with careful monitoring of Potassium levels 7.4.11.4.3 The maximum allowed rate of infusion on the Pediatric floor should not exceed 0.2 mmol/kg/hr (or 0.2 mEq/kg/hr, which are exactly the same) 7.4.11.4.4 Diluted Potassium Chloride > 40mEq/L via the central line can only be ordered by the Consultant PICU/PEC physician on service and must be double checked by overall in-charge nurse. No more than 80 mEql/L should be given via the central line, and the infusion should be only for maximum 2 hours after ensuring proper intravenous site assessment in a PICU setting and PEC only 7.4.11.4.5 The final concentration of a potassium infusion in the PICU via central line is 100-200 mEq/L (i.e., 0.1-0.2 mEq/mL) in exceptional circumstances 7.4.11.4.6 Solutions containing high potassium concentrations can cause skin sloughing and tissue necrosis. Use dextrose 5% in water or 0.9% sodium Chloride as diluent for Potassium chloride 7.4.11.4.7 Maximum rate of infusion in the PICU should not exceed 0.5 mmol/kg/hr (or 0.5 mEq/kg/hr, which are exactly the same) 7.4.11.4.8 Patients should be on a cardiac monitor in the ICU with any rate of infusion greater than 0.2 mmol/kg/hr 7.4.11.5 Precautions 7.4.11.5.1 Administer Potassium chloride infusion to maintain serum Potassium between 3.5 and 4.5 mEq/L. After infusion of one Potassium chloride, check serum Potassium level. 7.4.11.5.2 Always check and double check infusion rate on syringe pump and monitor Potassium chloride during entire infusion. Check the infusion rate on the infusion pump before start of the infusion and midpoint of the infusion 7.4.11.5.3 Ensure proper catheter or needle position prior to and during infusion to avoid extravasation and skin irritation 7.4.11.5.4 Do not infuse rapidly; high plasma concentrations of potassium may cause death due to cardiac depression, arrhythmias, or Governance, Leadership & Direction Corporate Clinical Practice Guidelines Committee APPENDIX B-2 CLINICAL PROTOCOL Management of pediatric patients with Hypokalemia TITLE: IDENTIFICATION NUMBER: CPRO 23043 HOSPITAL(S): All HMC Hospitals with Pediatric facilities (HGH, Al Wakrah, Al Khor, Cuban hospital) ORIGINAL DATE: October 2023 LAST REVISION DATE: October 2023 NEXT REVIEW DATE: October 2026 Sheet No. 7 of 11 arrest; plasma levels do not necessarily reflect tissue levels; monitor potassium replacement therapy 7.4.11.5.5 Higher dosages may increase risk of cardiac complications. 7.4.12 Consultation with Nephrology 7.4.12.1 Hypokalemia in a chronic case followed by nephrology at HMC. 7.4.12.2 Hypokalemia with associated hypertension, polyuria and dyselectrolytemia like hyponatremia, metabolic acidosis or metabolic alkalosis 7.4.13 Contact PICU for Patients with any of the following 7.4.13.1 Serum potassium <2.5 mmol/l. Some chronic cases may be handled on the floor (if stable) 7.4.13.2 Symptomatic hypokalemia of any level 7.4.13.3 ECG changes 7.4.13.4 Renal impairment 7.4.13.5 Risk of cardiovascular arrhythmia 7.4.13.6 Fluid overload 7.4.13.7 Complex children with renal, oncological, hematological, cardiac, endocrinological, and metabolic conditions 7.4.13.8 Start management in PEC/Floor in case of symptomatic hypokalemia with ECG changes and at the same time arrange for PICU admission 7.4.14 Consultation with Sidra Medicine: 7.4.14.1 Governance, Leadership & Direction If the child with hypokalemia visiting PEC is a chronic casefollowed by nephrology at Sidra, consult pediatric nephrology on call in Sidra from PEC Corporate Clinical Practice Guidelines Committee APPENDIX B-2 CLINICAL PROTOCOL Management of pediatric patients with Hypokalemia TITLE: IDENTIFICATION NUMBER: CPRO 23043 HOSPITAL(S): All HMC Hospitals with Pediatric facilities (HGH, Al Wakrah, Al Khor, Cuban hospital) ORIGINAL DATE: October 2023 LAST REVISION DATE: October 2023 NEXT REVIEW DATE: October 2026 Sheet No. 8 of 11 Key performance measures: Process Measure: • Percentage of patients with a potassium less than 3 mEq/L, who had an ECG requested and completed for them • Percentage of patients who received a potassium infusion greater than 0.2 meq/kg/hr and were placed under appropriate continuous cardiac monitoring in PICU or pediatric emergency Outcome Measure: • Percentage of patients who had skin irritation during potassium administration via peripheral line 8.0 Evidence-Based References: 8.1 Somers, M., & Traum, A. Hypokalemia in Children. In: UpToDate. Accessed on 22 July 2023 https://www.uptodate.com/contents/hypokalemia-in-children 8.2 Fluid and electrolyte management University Hospital of Leicester Children’s Hospital Guideline, United Kingdom. Approved by Children’s Clinical Practice Group, NHS on: September 2022 8.3 Sydney Children’s Hospital, Electrolyte Replacement Prescribing – SCH Practice Guideline. GuidelineNo:2018-036v1 Date of Publishing: 27 March 2018 12:24 PM Date of Access: 24 September 2023 https://www.schn.health.nsw.gov.au/_policies/pdf/2018-036.pdf 8.4 Evelina London. (2018). Pediatric Critical Care: Electrolyte Emergencies. Retrieved March 23, 2022, from https://www.evelinalondon.nhs.uk/resources/our-services/hospital/souththamesretrievalservice/electrolyte-emergencies-apr-2018.pdf 8.5 Wessex Paediatric Oncology Supportive Care Guidelines AM/CC/JG/RR/UU/JB 2016 Governance, Leadership & Direction Corporate Clinical Practice Guidelines Committee APPENDIX B-2 CLINICAL PROTOCOL Management of pediatric patients with Hypokalemia TITLE: IDENTIFICATION NUMBER: CPRO 23043 HOSPITAL(S): All HMC Hospitals with Pediatric facilities (HGH, Al Wakrah, Al Khor, Cuban hospital) ORIGINAL DATE: October 2023 LAST REVISION DATE: October 2023 NEXT REVIEW DATE: October 2026 Sheet No. 9 of 11 Authors: Dr. Shabina Khan - Consultant, General Pediatric Dr. Shital Mehta - Associate Consultant, Pediatric Nephrology Dr. Walid Abdulmalik Al Jbawi – Consultant, PICU Dr. Mohd Al Shaof -Consultant, Pediatric Emergency Center Dr. Samar Mahgoub – Consultant, General Pediatric Dr. Aimen Bashir -Consultant, PICU Dr. Mostafa Ahmed Ibrahim - Specialist, Pediatric Nephrology Nursing: Ms Jessy William PICU Ms Betty Jaison, Head Nurse -PEC Al Saad Reviewed by: Dr Bashir Youssef, Senior Consultant, PECs Dr Mohammed Al Kuwari – Consultant, Head for General Pediatrics Dr. Hossamaldein Gaber Mahgoub Ali – Clinical Pharmacist Approved by: Dr Mohammed Al Amri, Chairman of Pediatrics &Director of PECs Governance, Leadership & Direction Corporate Clinical Practice Guidelines Committee APPENDIX B-2 CLINICAL PROTOCOL Management of pediatric patients with Hypokalemia TITLE: IDENTIFICATION NUMBER: CPRO 23043 HOSPITAL(S): All HMC Hospitals with Pediatric facilities (HGH, Al Wakrah, Al Khor, Cuban hospital) ORIGINAL DATE: October 2023 LAST REVISION DATE: October 2023 NEXT REVIEW DATE: October 2026 Sheet No. 10 of 11 Appendix- 1 - Algorithm for Assessment of Hypokalemia Governance, Leadership & Direction Corporate Clinical Practice Guidelines Committee APPENDIX B-2 CLINICAL PROTOCOL Management of pediatric patients with Hypokalemia TITLE: IDENTIFICATION NUMBER: CPRO 23043 HOSPITAL(S): All HMC Hospitals with Pediatric facilities (HGH, Al Wakrah, Al Khor, Cuban hospital) ORIGINAL DATE: October 2023 LAST REVISION DATE: October 2023 NEXT REVIEW DATE: October 2026 Sheet No. 11 of 11 Appendix 2 Governance, Leadership & Direction Corporate Clinical Practice Guidelines Committee