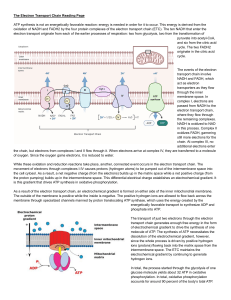
Biological oxidation Biological oxidation • The transfer of electrons from the reduced co- enzymes through the respiratory chain to oxygen is known as biological oxidation. • Energy released during this process is trapped as ATP. This coupling of oxidation with phosphorylation is called oxidative phosphorylation. • In the body, this oxidation is carried out by successive steps of dehydrogenations The seat for biological oxidation process inside a cell is the mitrochondria. There are enzymes that catalyze the oxidation process are called oxidoreductases. Different enzymes associated with biological oxidation are: 1. Oxidoreductases: These enzymes catalyse the removal of hydrogen from the substrate and add it to another substance, thus bringing about oxidation reduction reaction. Ex. Glyceraldehyde—3— Phosphate dehydrogenase. 2. Oxidases: These enzymes catalyse the removal of hydrogen from the substrate and add directly to the molecular oxygen. Ex. Cytochrome oxidases, tyrosinase, uricase. 3. Oxygenases: These enzymes incorporate oxygen into the substrates. (a) Mono-oxygenases: Adds only one atom of oxygen to the substrate. These are also known as mixed function oxidases. (b) Di-oxygenases: Adds both the atoms of oxygen to the substrate. Ex. Homogentisic acid di-oxygenase. 4. Aerobic dehydrogenases: These enzymes remove hydrogen from the substrate and add it either directly to oxygen or any other artificial acceptors like methylene blue. The product formed is hydrogen peroxide. 5. Anaerobic dehydrogenases: These enzymes use other substrates or substances to donate the hydrogen. They transfer hydrogen’s to some other hydrogen acceptor, but not directly to oxygen. Thus the hydrogen acceptors are NAD, FAD and FMN. Heme proteins like cytochromes also receive hydrogen’s. The cytochromes are ‘b’, ‘c1‘, ‘c’, ‘a’ and 'a3' 6. Hydro peroxidases: These enzymes have either hydrogen peroxide (H2O2) or organic peroxide as their substrate. There are two types of hydro peroxidases: (1) Peroxidase and (2) Catalase. Their prime function is destruction of H2O2. Organization of electron transport chain Electron transport chains (ETC) take place in the inner membrane of the mitochondrion. . In this stage, energy from NADH and FADH2, is transferred to AT: 1- High-energy electrons are released from NADH and FADH2, and they move along ETC. 2- The electrons combine with Oxygen to form Water. 3- As the high-energy electrons are transported along ETC some of their energy is captured. This energy is used to pump H+ across the inner membrane (from the matrix into the intermembrane space). 4- Because of high amount of hydrogen A chemiosmotic gradient causes hydrogen ions to flow back across the mitochondrial membrane into the matrix through ATP synthase enzyme, producing ATP The electron transport chain in the mitochondrion is the site of oxidative phosphorylation in eukaryotes. It mediates the reaction between NADH or succinate generated in the citric acid cycle and oxygen to power ATP synthase The flow of electrons through the electron transport chain is an exergonic process. The energy from the redox reactions creates an electrochemical proton gradient that drives the synthesis of adenosine triphosphate (ATP). In aerobic respiration, the flow of electrons terminates with molecular oxygen as the final electron acceptor. In anaerobic respiration, other electron acceptors are used, such as sulfate. In an electron transport chain, the redox reactions are driven by the difference in the Gibbs free energy of reactants and products. The free energy released when a higher-energy electron donor and acceptor convert to lower-energy products, while electrons are transferred from a lower to a higher redox potential, is used by the complexes in the electron transport chain to create an electrochemical gradient of ions. It is this electrochemical gradient that drives the synthesis of ATP via coupling with oxidative phosphorylation with ATP synthase. In eukaryotic organisms the electron transport chain, and site of oxidative phosphorylation, is found on the inner mitochondrial membrane. The energy released by reactions of oxygen and reduced compounds such as cytochrome c and (indirectly) NADH and FADH2 is used by the electron transport chain to pump protons into the intermembrane space, generating the electrochemical gradient over the inner mitochondrial membrane. In photosynthetic eukaryotes, the electron transport chain is found on the thylakoid membrane. Here, light energy drives electron transport through a proton pump and the resulting proton gradient causes subsequent synthesis of ATP. In bacteria, the electron transport chain can vary between species but it always constitutes a set of redox reactions that are coupled to the synthesis of ATP through the generation of an electrochemical gradient and oxidative phosphorylation through ATP synthase The final stage of cellular respiration is oxidative phosphorylation that consists of two steps: the electron transport chain and chemiosmosis. The electron transport chain is a set of proteins found in the inner mitochondrial membrane in eukaryotic cells. Its primary function is to establish a proton gradient that can be used during chemiosmosis to produce ATP and generate electron carriers, such as NAD+ and FAD, that are used in glycolysis and the citric acid cycle. The ETC is comprised of protein complex I, II, III, and IV. NADH and FADH2 are reduced electron carriers that donate electrons to the ETC complexes. NADH can directly donate electrons into complex I, while FADH2 donates electrons into complex II. Upon donation of electrons, NADH and FADH2 are converted back to their oxidized forms NAD+ and FAD, respectively. These ETC complexes pass electrons to one another through multiple redox reactions in an energetically downhill sequence. These reactions release energy that is used to pump H+ across the inner membrane from the matrix into the intermembrane space, establishing a proton gradient across the inner membrane. The flow of H+ ions down their electrochemical gradient back into the matrix through ATP synthase enables the conversion of ADP to ATP. Reactions of electron transport chain