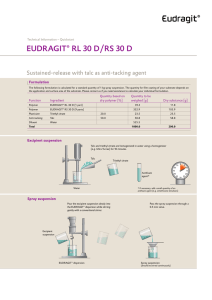
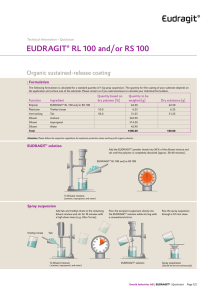
Technical Information EUDRAGIT® L 100-55 EIP / Product Regulatory Datasheet …………………………………………………………………………... This Product Regulatory Data Sheet is built up following the standardized Excipient Information Package (EIP) format published by IPEC (International Pharmaceutical Excipients Council). Table of Contents 1 General Product Information 2 Manufacturing, Packaging and Release Site Information 3 Physico-Chemical Information 4 Regulatory Information 5 Miscellaneous Product Information 6 Revisions 7 Contact Information 1 General Product Information Product: EUDRAGIT® L 100-55. Scope: This datasheet pertains only to the product listed above. Specification and testing methods: see product specification INFO 7.4/E. Storage conditions: see product specification INFO 7.4/E and product label. Minimum stability date: see certificate of analysis and product label. EIP - L 100-55 Evonik Operations GmbH EUDRAGIT® L 100-55 July 2020 Page 1/11 2 Manufacturing, Packaging and Release Site Information EUDRAGIT® L 100-55 is manufactured, tested for release, packaged, labeled and stored under the responsibility of the following company: Evonik Operations GmbH Kirschenallee 64293 Darmstadt (Germany) Telephone: Fax: Homepage: +49 (0) 6151 18 01 +49 (0) 6151 18 02 http://www.eudragit.com Interim Storage Qualified third party logistic specialists in Germany may be used for the interim storage of this product. This product is manufactured in conformance with: GMP standard: Management system: ESHQ policy: EXCIPACT™ certification 3 The Joint IPEC – PQG Good Manufacturing Practice Guide for Pharmaceutical Excipients 2017 and USP-NF General Chapter <1078> Quality: ISO 9001 Environment: ISO 14001 see ESHQ values: http://www.evonik.com (Responsibility) Evonik Pharma Polymers & Services is certified since January 2014 Physico-Chemical Information CAS number 25212-88-8 FDA UNII NX76LV5T8J Chemical/IUPAC Name Poly(methacrylic acid-co-ethyl acrylate) 1:1. INCI Name Acrylates Copolymer. FDA-SRS Preferred Substance Term Methacrylic acid - ethyl acrylate copolymer (1:1) type A. EIP - L 100-55 Evonik Operations GmbH EUDRAGIT® L 100-55 July 2020 Page 2/11 Physical Properties EUDRAGIT® L 100-55 is a solid substance in form of a white powder with a faint characteristic odor. Molar Mass Information MW approx. 320,000 g/mol. In previous publication the approximate weight average molar mass was indicated to be 250,000. This was determined by viscometry in the 1960ies and had never been re-evaluated since then. The determination of the weight average molar mass of acrylic copolymer by size exclusion chromatography (SEC) or gel permeation chromatography (GPC), respectively, is difficult due to adsorptive and associative phenomena of these polymers. In 2004 a robust SEC method was developed for this polymer. Based on this method the weight average molar mass is approximately MW 320,000 g/mol. Chemical Properties EUDRAGIT® L 100-55 contains an anionic copolymer based on methacrylic acid and ethyl acrylate. The ratio of the free carboxyl groups to the ester groups is approximately 1:1. The product contains 0.7 % Sodium Laurylsulfate Ph. Eur. / NF and 2.3 % Polysorbate 80 Ph. Eur. / NF on solid substance. EUDRAGIT® L 100-55 is the dry substance obtained from EUDRAGIT® L 30 D-55 by spray drying. Chemical Structure The monomers are statistically ordered in the copolymer chain. Production Process Emulsion polymerization and subsequent spray drying. EIP - L 100-55 Evonik Operations GmbH EUDRAGIT® L 100-55 July 2020 Page 3/11 Product Flow EIP - L 100-55 Evonik Operations GmbH EUDRAGIT® L 100-55 July 2020 Page 4/11 4 Regulatory Information Compendial Compliance EUDRAGIT® L 100-55 meets the specifications of the following pharmacopoeias: Ph. Eur. Methacrylic Acid - Ethyl Acrylate Copolymer (1:1) Type A USP-NF Methacrylic Acid and Ethyl Acrylate Copolymer JPE Dried Methacrylic Acid Copolymer LD Drug Master File EUDRAGIT® L 100-55 is described in US Drug Master File: # 002584. A Letter of Authorization is issued upon written request. Food Label USA: not approved as direct or as indirect food additive (21 CFR 172 to 177 and 180 to 186) EU: not listed (Council Directive 89/107/EEC and amendments) Production Related Substances Sulfur dioxide and sulphites Disinfectant additives Preservatives Plasticizers (e.g. phthalates) Emulsifiers Simethicone Antioxidants Sodium chloride Iodine and iodine salts EIP - L 100-55 Not anticipated to be more than 10 mg/kg No No No 0.7 % sodium laurylsulfate and 2.3 % polysorbate 80 with ref. to dry subst. Minute amounts of a silicone antifoaming emulsion are used in the production process No No No Evonik Operations GmbH EUDRAGIT® L 100-55 July 2020 Page 5/11 Allergen and Compound Ingredient Disclosure Statement The allergens named in EU Directive 1169/2011 and relevant amendments are not used in the manufacture of EUDRAGIT® L 100-55. The product does not contain any of the sensitizing fragrances listed in Annex III of EU Regulation 1223/2009 entries 67 to 92, in the Appendix of the Annex to the EC Guideline "Excipients in the labelling and package leaflet of medicinal products for human use" and in Australian TGO 91/92. EUDRAGIT® products are synthetic acrylic copolymers. The following substances are neither used nor intentionally added in the manufacture and packaging of EUDRAGIT® products. Therefore, more than omnipresent traces are not anticipated in the product. In addition, none of the potent substances indicated with an asterisk (*) are produced or handled in the same production site either. Abalone Grapes & grapevine derivatives Amino acids (e.g. glutamate, LHerbicides (*) phenylalanine) Animal Coal Honey Animal derived products and animal-byHormones (*) products Antibiotics (e.g. penicillin) (*) Human derived products Artificial colours Lactose Artificial flavors Latex and latex derivatives Artificial sweetener Lupine Aristolochic acid Malt dextrin Asbestos Mesylate ester Beef and beef derivatives Milk Bisphenol (A-Z) Molluscs Bovine and bovine products Mustard Caffeine Nuts (almonds, hazelnuts, walnuts, cashews, pecan nuts, Brazil nuts, pistachio nuts, macadamia nuts, Queensland nuts) Carbohydrates Peanuts and peanut products Carbomer homopolymer Pesticides (*) Casein Pork and pork products Celery and celery products Proteins Cellulose Radioactive substances (*) Cereals (corn, buckwheat, wheat, rye, barley, oats spelt, kamut or other hybridized Rice and rice products strains) Chicken and chicken products Rosin Cochineal extract Sesame seeds Collagen Sorbitol Crustaceans Soy and soy products (soybeans) Cytotoxics (*) Squid Dairy products and derivatives Starch Eggs and egg products Stem cells or eukaryotic cells Fish and fish products Steroids (*) Flaxseed Sugar Fragrances Sugar alcohol (e.g. xylitol, maltitol) Fructose Sulphonic acid ester Gelatin Table salt Gluten (corn, wheat, oat, rye) Wool derivatives Glycerin Yeast / autolyzed yeast EIP - L 100-55 Evonik Operations GmbH EUDRAGIT® L 100-55 July 2020 Page 6/11 Residual Solvents Organic solvents are not used in the manufacture, packing and storage of this product. Small amounts of ethanol may be detectable within the minimum stability period. The concentration remains below 0.5 %. Source: The ethyl acrylate integrated in polymer chain may hydrolyze chemically at a very low level to acrylic acid and ethanol. The acrylic acid remains in the polymer chain but ethanol is released. This hydrolysis starts with manufacturing and is ongoing during storage. A Residual Solvents Statement is given on every Certificate of Analysis on page 2. BSE/TSE Status The product covered by this document is synthetically manufactured in equipment restricted to the production of chemical products in facilities where no animal derived materials are used. Based on the manufacturing and processing methods used there is no potential for TSE/BSE agent to be present in the product. A TSE/BSE Confirmation/Statement is given on every Certificate of Analysis on page 2. Genetically Modified Material (GMM) / Genetically Modified Organism (GMO) Information This product is manufactured by chemical process. Certain ingredients, specifically, Sodium Laurylsulfate Ph. Eur. / NF and Polysorbate 80 Ph. Eur. / NF are of natural origin. Our suppliers confirmed that genetically modified materials are not used in the manufacturing of these ingredients. A GMM/GMO statement is given on every Certificate of Analysis on page 2. Melamine Information Animal derived products or nitrogen-containing raw materials including melamine are neither used in the manufacture nor packaging of EUDRAGIT® products. Consequently EUDRAGIT® products are not tested for melamine content. More than omnipresent traces are not anticipated in the product. Nitrosamine Information Nitrosamine information is available upon request. Sodium/Potassium/Calcium/Magnesium Not more than 0.2 % each in the product calculated from the limit for sulphated ash (max. 0.4 %). Elemental Impurities and Heavy Metals Elemental Impurities and Heavy Metals information is provided in the specification. Background information is available upon request. Kosher/Halal Status Neither Kosher nor Halal certificates are available. The product is manufactured by chemical synthesis. No animal derived raw materials are used. Halal: poly acrylic acid and its salts are considered as Halal according to Indonesian authority LPPOM MUI (document# SK07.I.2013). EIP - L 100-55 Evonik Operations GmbH EUDRAGIT® L 100-55 July 2020 Page 7/11 Viral Safety Neither animal/human cells nor human/animal derived materials are used in the manufacture of this product. Therefore viral contaminations are not anticipated in this product. Bioburden/Pyrogens The product is a synthetic (meth)acrylic copolymer. Microbial purity is controlled and specified (see specification). A bioburden or pyrogenic contamination is not anticipated in the product. Aflatoxin The product is a synthetic (meth)acrylic copolymer. Microbial purity is controlled and specified (see specification). An aflatoxin contamination is not anticipated in the product. Dioxin Dioxin is neither used in the manufacture nor anticipated from raw materials nor from production conditions. Therefore dioxin contaminations are not anticipated in this product. Irradiation Neither the product nor the raw materials are subjected to irradiation. Proposition 65 See product US Material Safety Data Sheet. 5 Miscellaneous Product Information Batch Definition A batch is a quantity of homogenous product manufactured either within a manufacturing cycle or during a defined time period. Batch/Lot Numbering System The lot number is composed of a letter at the first place which represents an internal production site, followed then by nine digits representing a manufacturing order date, product code and sequential batch number. Packaging Material The materials used in the manufacture of EUDRAGIT® primary packaging materials are allowed according to EU food packaging legislation. EIP - L 100-55 Evonik Operations GmbH EUDRAGIT® L 100-55 July 2020 Page 8/11 Re-evaluation Period EUDRAGIT® L 100-55 is stable in the original sealed and unopened container stored under conditions as specified for at least 36 months. The minimum stability date is given on the label of each individual container and on each Certificate of Analysis on page 1. If the product is to be used after longer storage we recommend performing tests given in the 'EUDRAGIT® Products suggested tests for Re-examination' leaflet. Especially in case of advanced formulations we recommend to consider if a trial production and suitability tests are necessary. Technically Unavoidable Particles Profile (TUPP) TUPP information is available upon request. History of Use Year of introduction to the market: 1985. EUDRAGIT® L 100-55 is the dry substance obtained from EUDRAGIT® L 30 D-55. The polymer dispersion EUDRAGIT® L 30 D-55 is commercially available since 1972. Common Uses EUDRAGIT® polymers are widely used as a platform for functional oral solid dosage forms. Macronutrient Information The polymer is neither absorbed nor degraded in the gastro-intestinal system. It is excreted unchanged. Safety Information Summary of safety data is available upon request. Stability Information Storage stability data is available upon request. 6 Revisions Date of Publication Document Version July 2015 6.0 June 2018 July 2020 EIP - L 100-55 Revision Description Update: Company name change, new USP-NF monograph name mandatory Dec 1, 2015, minor modifications on existing texts 7.0 Update: minor editorial modifications, interim storage, SRS name, Elemental Impurities, EU food packaging legislation, common uses 8.0 Update: Company name change, minor editorial modifications, Australian TGO 91/92, Nitrosamine, TUPP Evonik Operations GmbH EUDRAGIT® L 100-55 July 2020 Page 9/11 7 Contact Information Regulatory Affairs (Evonik Operations GmbH) Dr. Johanna Eisele Head of Regulatory Affairs Tel: +49 6151 18-4763 E-mail: johanna.eisele@evonik.com Quality Assurance (Evonik Operations GmbH) Mr. Boris Glasbrenner Head QA Operations Tel: +49 6151 18-4879 E-mail: boris.glasbrenner@evonik.com Sales (Evonik Operations GmbH) Mr. Karsten Weber Head of Global Sales Support Tel: +49 6151 18-3550 E-mail: karsten.weber@evonik.com USA Contact (Evonik Corporation) Mr. Chris Armstrong Director Supply / Demand Chain Tel: +1 732 981-5339 E-mail: chris.armstrong@evonik.com Date: July 2020 Dr. Johanna Eisele Boris Glasbrenner Evonik Operations GmbH Head of Regulatory Affairs Pharma Polymers & Services Evonik Operations GmbH Head QA Operations Darmstadt/Weiterstadt Health Care (written by computer and therefore not signed) '100120927 - TBBT! EIP - L 100-55 Evonik Operations GmbH EUDRAGIT® L 100-55 July 2020 Page 10/11 This information and all further technical advice are based on our present knowledge and experience. However, it implies no liability or other legal responsibility on our part, including with regard to existing third party intellectual property rights, especially patent rights. In particular, no warranty, whether expressed or implied, or guarantee of product properties in the legal sense is intended or implied. We reserve the right to make any changes according to technological progress or further developments. The customer is not released from the obligation to conduct careful inspection and testing of incoming goods. Performance of the product described herein should be verified by testing, which should be carried out only by qualified experts in the sole responsibility of a customer. Reference to trade names used by other companies is neither a recommendation, nor does it imply that similar products could not be used. ® = registered trademark The name EUDRAGIT® is a protected trademark owned by Evonik Industries or one of its subsidiaries July 2020 Evonik Operations GmbH Kirschenallee, 64293 Darmstadt, Germany PHONE +49 6151 18-4019, FAX +49 6151 18-3520 www.eudragit.com EIP - L 100-55 Evonik Operations GmbH EUDRAGIT® L 100-55 July 2020 Page 11/11