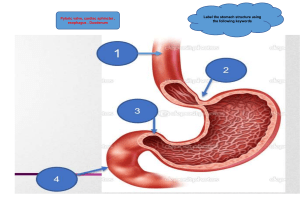
25 chapter Surgical Anatomy Physiology 1009 1015 Swallowing Mechanism / 1015 Physiologic Reflux / 1017 Assessment of Esophageal Function 1018 Tests to Detect Structural Abnormalities / 1018 Tests to Detect Functional Abnormalities / 1019 Video- and Cineradiography / 1028 Tests to Detect Increased Exposure to Gastric Juice / 1028 Tests of Duodenogastric Function / 1030 Gastroesophageal Reflux Disease 1031 The Human Antireflux Mechanism and the Pathophysiology of Gastroesophageal Reflux Disease / 1032 Complications Associated With Gastroesophageal Reflux Disease / 1033 Metaplastic (Barrett’s Esophagus) and Neoplastic (Adenocarcinoma) Complications / 1035 Respiratory Complications / 1035 Surgical Therapy for Gastroesophageal Reflux Disease / 1038 Primary Antireflux Repairs / 1040 Giant Diaphragmatic (Hiatal) Hernias Incidence and Etiology / 1045 Clinical Manifestations / 1047 Diagnosis / 1047 Pathophysiology / 1048 Treatment / 1048 1045 Esophagus and Diaphragmatic Hernia Blair A. Jobe, John G. Hunter, and David I. Watson Diaphragmatic Repair / 1048 The Short Esophagus and PEH / 1049 Results / 1049 Schatzki’s Ring Scleroderma Eosinophilic Esophagitis 1049 1050 1051 Symptoms / 1051 Signs / 1051 Pathology / 1051 Treatment / 1051 Motility Disorders of the Pharynx and Esophagus 1052 Clinical Manifestations / 1052 Motility Disorders of the Pharynx and Upper Esophagus—Transit Dysphagia / 1052 Diagnostic Assessment of the Cricopharyngeal Segment / 1052 Motility Disorders of the Esophageal Body and Lower Esophageal Sphincter / 1055 Operations for Esophageal Motor Disorders and Diverticula 1060 Long Esophageal Myotomy for Motor Disorders of the Esophageal Body / 1060 Myotomy of the Lower Esophageal Sphincter (Heller Myotomy) / 1063 Open Esophageal Myotomy / 1065 Laparoscopic Cardiomyotomy / 1065 Per Oral Endoscopic Myotomy (POEM) / 1065 Outcome Assessment of the Therapy for Achalasia / 1065 Esophageal Resection for End-Stage Motor Disorders of the Esophagus / 1068 SURGICAL ANATOMY The esophagus is a muscular tube that starts as the continuation of the pharynx and ends as the cardia of the stomach. When the head is in a normal anatomic position, the transition from pharynx to esophagus occurs at the lower border of the sixth cervical vertebra. Topographically this corresponds to the cricoid cartilage anteriorly and the palpable transverse process of the sixth cervical vertebra laterally (Fig. 25-1). The esophagus is firmly attached at its upper end to the cricoid Carcinoma of the Esophagus 1068 Clinical Manifestations / 1068 General Approach to Esophageal Cancer / 1069 Staging of Esophageal Cancer / 1069 Clinical Approach to Carcinoma of the Esophagus and Cardia / 1070 Palliation of Esophageal Cancer / 1074 Surgical Treatment / 1074 Comparative Studies of Esophagectomy Technique / 1077 Alternative Therapies / 1077 Sarcoma of the Esophagus Benign Tumors and Cysts 1078 1080 Leiomyoma / 1081 Esophageal Cyst / 1083 Esophageal Perforation 1083 Diagnosis / 1083 Management / 1084 Mallory-Weiss Syndrome Caustic Injury 1085 1086 Pathology / 1086 Clinical Manifestations / 1086 Treatment / 1086 Acquired Fistula Techniques of Esophageal Reconstruction 1088 1089 Partial Esophageal Resection / 1089 Reconstruction After Total Esophagectomy / 1089 Composite Reconstruction / 1090 Vagal Sparing Esophagectomy With Colon Interposition / 1090 cartilage and at its lower end to the diaphragm; during swallowing, the proximal points of fixation move craniad the distance of one cervical vertebral body. The esophagus lies in the midline, with a deviation to the left in the lower portion of the neck and upper portion of the thorax, and returns to the midline in the midportion of the thorax near the bifurcation of the trachea (Fig. 25-2). In the lower portion of the thorax, the esophagus again deviates to the left and anteriorly to pass through the diaphragmatic hiatus. Key Points 1 Barrett’s esophagus is the transformation of the distal esophageal epithelium from squamous to a specialized columnar epithelium capable of further neoplastic progression. The detection of Barrett’s esophagus on endoscopy and biopsy increases the future risk of cancer by >40x compared to individuals without Barrett’s esophagus. Giant hiatal hernia, otherwise known as paraesophageal hernia, should be repaired when symptomatic or associated with iron deficiency anemia. Laparoscopic hiatal hernia repair with fundoplication is the most common approach to repair. Achalasia is the most common primary esophageal motor disorder. It is characterized by an absence of peristalsis and a hypertensive nonrelaxing lower esophageal sphincter. It is best treated with laparoscopic Heller myotomy and partial fundoplication. Most esophageal cancer presents with dysphagia, at which time it has invaded the muscularis of the esophagus and is often associated with lymph node metastases. The preferred treatment at this stage is multimodality therapy with chemoradiation therapy followed by open or minimally invasive esophagectomy. Three normal areas of esophageal narrowing are evident on the barium esophagogram or during esophagoscopy. The uppermost narrowing is located at the entrance into the esophagus and is caused by the cricopharyngeal muscle. Its luminal diameter is 1.5 cm, and it is the narrowest point of the esophagus. The middle narrowing is due to an indentation of the anterior and left lateral esophageal wall caused by the crossing of the left main stem bronchus and aortic arch. The luminal diameter at this point is 1.6 cm. The lowermost narrowing is at the hiatus of the diaphragm and is caused by the gastroesophageal sphincter mechanism. The luminal diameter at this point varies somewhat, depending on the distention of the esophagus by the passage of food, but has been measured at 1.6 to 1.9 cm. These normal constrictions tend to hold up swallowed foreign objects, and the overlying mucosa is subject to injury by swallowed corrosive liquids due to their slow passage through these areas. Figure 25-3 shows the average distance in centimeters measured during endoscopic examination between the incisor teeth and the cricopharyngeus, aortic arch, and cardia of the stomach. Manometrically, the length of the esophagus between the lower border of the cricopharyngeus and upper border of the lower sphincter varies according to the height of the individual. 2 3 Benign esophageal disease is common and is best evaluated with thorough physiologic testing (high resolution esophageal motility, 24-hour ambulatory pH measurement, and/or esophageal impedance testing) and anatomic testing (esophagoscopy, video esophagography, and/or computed tomography [CT] scanning). Gastroesophageal reflux disease (GERD) is the most common disease of the gastrointestinal tract for which patients seek medical therapy. When GERD symptoms (heartburn, regurgitation, chest pain, and/or supraesophageal symptoms) are troublesome despite adequately dosed PPI, surgical correction may be indicated. a b c d e A 1010 B 4 5 6 Figure 25-1. A. Topographic relationships of the cervical esophagus: (a) hyoid bone, (b) thyroid cartilage, (c) cricoid cartilage, (d) thyroid gland, (e) sternoclavicular. B. Lateral radio-graphic appearance with landmarks identified as labeled in A. The location of C6 is also included (f). (Reproduced with permission from Shields TW: General Thoracic Surgery, 3rd ed. Philadelphia, PA: Lea & Febiger; 1989.) 1011 Figure 25-2. Barium esophagogram. A. Posterior-anterior view. White arrow shows deviation to left. Black arrow shows return to midline. B. Lateral view. Black arrow shows anterior deviation. (Reproduced with permission from Shields TW: General Thoracic Surgery, 3rd ed. Philadelphia, PA: Lea & Febiger; 1989.) Incisor teeth Pharynx 15cm 14cm The pharyngeal musculature consists of three broad, flat, overlapping fan-shaped constrictors (Fig. 25-4). The opening of the esophagus is collared by the cricopharyngeal muscle, which arises from both sides of the cricoid cartilage of the larynx and forms a continuous transverse muscle band without an interruption by a median raphe. The fibers of this muscle 24–26cm Upper sphincter (C6) Superior pharyngeal constrictor m. Aortic arch (T4) 40cm 38cm Middle pharyngeal constrictor m. 25cm 23cm Inferior pharyngeal constrictor m. Cricopharyngeus m. Lower sphincter (T11) Esophagus A Figure 25-3. Important clinical endoscopic measurements of the esophagus in adults. (Reproduced with permission from Shields TW: General Thoracic Surgery, 3rd ed. Philadelphia, PA: Lea & Febiger; 1989.) B Figure 25-4. External muscles of the pharynx. A. Posterolateral view. B. Posterior view. Dotted line represents usual site of myotomy. (Reproduced with permission from Shields TW: General Thoracic Surgery, 3rd ed. Philadelphia, PA: Lea & Febiger; 1989.) CHAPTER 25 ESOPHAGUS AND DIAPHRAGMATIC HERNIA B A 1012 PART II SPECIFIC CONSIDERATIONS blend inseparably with those of the inferior pharyngeal constrictor above and the inner circular muscle fibers of the esophagus below. Some investigators believe that the cricopharyngeus is part of the inferior constrictor; that is, that the inferior constrictor has two parts, an upper or retrothyroid portion having diagonal fibers, and a lower or retrocricoid portion having transverse fibers. Keith in 1910 showed that these two parts of the same muscle serve totally different functions. The retrocricoid portion serves as the upper sphincter of the esophagus and relaxes when the retrothyroid portion contracts, to force the swallowed bolus from the pharynx into the esophagus. The cervical portion of the esophagus is approximately 5 cm long and descends between the trachea and the vertebral column, from the level of the sixth cervical vertebra to the level of the interspace between the first and second thoracic vertebrae posteriorly, or the level of the suprasternal notch anteriorly. The recurrent laryngeal nerves lie in the right and left grooves between the trachea and the esophagus. The left recurrent nerve lies somewhat closer to the esophagus than the right, owing to the slight deviation of the esophagus to the left, and the more lateral course of the right recurrent nerve around the right subclavian artery. Laterally, on the left and right sides of the cervical esophagus are the carotid sheaths and the lobes of the thyroid gland. The thoracic portion of the esophagus is approximately 20 cm long. It starts at the thoracic inlet. In the upper portion of the thorax, it is in intimate relationship with the posterior wall of the trachea and the prevertebral fascia. Just above the tracheal bifurcation, the esophagus passes to the right of the aorta. This anatomic positioning can cause a notch indentation in its left lateral wall on a barium swallow radiogram. Immediately below this notch, the esophagus crosses both the bifurcation of the trachea and the left main stem bronchus, owing to the slight deviation of the terminal portion of the trachea to the right by the aorta (Fig. 25-5). From there down, the esophagus passes over the posterior surface of the subcarinal lymph nodes (LNs), and then descends over the pericardium of the left atrium to reach the diaphragmatic hiatus (Fig. 25-6). From the bifurcation of the trachea downward, Ascending aorta both the vagal nerves and the esophageal nerve plexus lie on the muscular wall of the esophagus. Dorsally, the thoracic esophagus follows the curvature of the spine and remains in close contact with the vertebral bodies. From the eighth thoracic vertebra downward, the esophagus moves vertically away from the spine to pass through the hiatus of the diaphragm. The thoracic duct passes through the hiatus of the diaphragm on the anterior surface of the vertebral column behind the aorta and under the right crus. In the thorax, the thoracic duct lies dorsal to the esophagus between the azygos vein on the right and the descending thoracic aorta on the left. The abdominal portion of the esophagus is approximately 2 cm long and includes a portion of the lower esophageal sphincter (LES). It starts as the esophagus passes through the diaphragmatic hiatus and is surrounded by the phrenoesophageal membrane, a fibroelastic ligament arising from the subdiaphragmatic fascia as a continuation of the transversalis fascia lining the abdomen (Fig. 25-7). The upper leaf of the membrane attaches itself in a circumferential fashion around the esophagus, about 1 to 2 cm above the level of the hiatus. These fibers blend in with the elastic-containing adventitia of the abdominal esophagus and the cardia of the stomach. This portion of the esophagus is subjected to the positive-pressure environment of the abdomen. The musculature of the esophagus can be divided into an outer longitudinal and an inner circular layer. The upper 2 to 6 cm of the esophagus contains only striated muscle fibers. From then on, smooth muscle fibers gradually become more abundant. Most clinically significant esophageal motility disorders involve only the smooth muscle in the lower two-thirds of the esophagus. When a long surgical esophageal myotomy is indicated, the incision needs to extend only this distance. The longitudinal muscle fibers originate from a cricoesophageal tendon arising from the dorsal upper edge of the anteriorly located cricoid cartilage. The two bundles of muscle diverge and meet in the midline on the posterior wall of the esophagus about 3 cm below the cricoid (see Fig. 25-4). From this point on, the entire circumference of the esophagus is Thymus Left main stem bronchus Pericardium Superior vena cava Bottom of aortic arch a e Tracheal carina Descending aorta b c d Right main stem bronchus IV Esophagus B A Figure 25-5. A. Cross-section of the thorax at the level of the tracheal bifurcation. B. Computed tomographic scan at same level viewed from above: (a) ascending aorta, (b) descending aorta, (c) tracheal carina, (d) esophagus, (e) pulmonary artery. (Reproduced with permission from Shields TW: General Thoracic Surgery, 3rd ed. Philadelphia, PA: Lea & Febiger; 1989.) 1013 Pericardium Right ventricle Left ventricle f Right atrium Left atrium d e Pericardium Esophagus c Pleura Aorta g b a VII B A Figure 25-6. A. Cross-section of the thorax at the midleft atrial level. B. Computed tomographic scan at same level viewed from above: (a) aorta, (b) esophagus, (c) left atrium, (d) right atrium, (e) left ventricle, (f) right ventricle, (g) pulmonary vein. (Reproduced with permission from Shields TW: General Thoracic Surgery, 3rd ed. Philadelphia, PA: Lea & Febiger; 1989.) covered by a layer of longitudinal muscle fibers. This configuration of the longitudinal muscle fibers around the most proximal part of the esophagus leaves a V-shaped area in the posterior wall covered only with circular muscle fibers. Contraction of the longitudinal muscle fibers shortens the esophagus. The circular muscle layer of the esophagus is thicker than the outer longitudinal layer. In situ, the geometry of the circular muscle is helical and makes the peristalsis of the esophagus assume a wormlike drive, as opposed to segmental and sequential squeezing. As a consequence, severe motor abnormalities of the esophagus assume a corkscrew-like pattern on the barium swallow radiogram. The cervical portion of the esophagus receives its main blood supply from the inferior thyroid artery. The thoracic portion receives its blood supply from the bronchial arteries, with 75% of individuals having one right-sided and two left-sided branches. Two esophageal branches arise directly from the aorta. The abdominal portion of the esophagus receives its blood supply from the ascending branch of the left gastric artery and from inferior phrenic arteries (Fig. 25-8). On entering the wall of the esophagus, the arteries assume a T-shaped division to form a longitudinal plexus, giving rise to an intramural vascular network in the muscular and submucosal layers. As a consequence, the esophagus can be mobilized from the stomach to the level of the aortic arch without fear of devascularization and ischemic necrosis. Caution, however, should be exercised as to the extent of esophageal mobilization in patients who have had a previous thyroidectomy with ligation of the inferior thyroid arteries proximal to the origin of the esophageal branches. Blood from the capillaries of the esophagus flows into a submucosal venous plexus, and then into a periesophageal Esophageal branch Inferior thyroid artery Right bronchial artery Diaphragm Phreno-esophageal membrane (Descending leaf) Superior left bronchial artery Inferior left bronchial artery Aortic esophageal arteries Phreno-esophageal membrane (Ascending leaf) Parietal peritoneum Visceral peritoneum Para-esophageal fat pad Ascending branches of left gastric artery Left gastric artery Figure 25-7. Attachments and structure of the phrenoesophageal membrane. Transversalis fascia lies just above the parietal peritoneum. (Reproduced with permission from Shields TW: General Thoracic Surgery, 3rd ed. Philadelphia, PA: Lea & Febiger; 1989.) Figure 25-8. Arterial blood supply of the esophagus. (Reproduced with permission from Shields TW: General Thoracic Surgery, 3rd ed. Philadelphia, PA: Lea & Febiger; 1989.) CHAPTER 25 ESOPHAGUS AND DIAPHRAGMATIC HERNIA Pleura 1014 PART II SPECIFIC CONSIDERATIONS venous plexus from which the esophageal veins originate. In the cervical region, the esophageal veins empty into the inferior thyroid vein; in the thoracic region, they empty into the bronchial, azygos, or hemiazygos veins; and in the abdominal region, they empty into the coronary vein (Fig. 25-9). The submucosal venous networks of the esophagus and stomach are in continuity with each other, and, in patients with portal venous obstruction, this communication functions as a collateral pathway for portal blood to enter the superior vena cava via the azygos vein. The parasympathetic innervation of the pharynx and esophagus is provided mainly by the vagus nerves. The constrictor muscles of the pharynx receive branches from the pharyngeal plexus, which is on the posterior lateral surface of the middle constrictor muscle, and is formed by pharyngeal branches of the vagus nerves with a small contribution from cranial nerves IX and XI (Fig. 25-10). The cricopharyngeal sphincter and the cervical portion of the esophagus receive branches from both recurrent laryngeal nerves, which originate from the vagus nerves—the right recurrent nerve at the lower margin of the subclavian artery and the left at the lower margin of the aortic arch. They are slung dorsally around these vessels and ascend in the groove between the esophagus and trachea, giving branches to each. Damage to these nerves interferes not only with the function of the vocal cords but also with the function of the cricopharyngeal sphincter and the motility of the cervical esophagus, predisposing the individual to pulmonary aspiration on swallowing. Afferent visceral sensory pain fibers from the esophagus end without synapse in the first four segments of the thoracic spinal cord, using a combination of sympathetic and vagal pathways. These pathways are also occupied by afferent visceral sensory fibers from the heart; hence, both organs have similar symptomatology. The lymphatics located in the submucosa of the esophagus are so dense and interconnected that they constitute a single Right vagus nerve Recurrent laryngeal nerves Left vagus nerve Right recurrent laryngeal nerve Left recurrent laryngeal nerve Anterior esophageal plexus Thoracic chain Left or anterior vagal trunk Right or posterior vagal trunk Figure 25-10. Innervation of the esophagus. (Reproduced with permission from Shields TW: General Thoracic Surgery, 3rd ed. Philadelphia, PA: Lea & Febiger; 1989.) plexus (Fig. 25-11). There are more lymph vessels than blood capillaries in the submucosa. Lymph flow in the submucosal plexus runs in a longitudinal direction, and, on injection of a contrast medium, the longitudinal spread is seen to be about six times that of the transverse spread. In the upper two-thirds of the esophagus, the lymphatic flow is mostly cephalad, and, in the lower third, caudad. In the thoracic portion of the esophagus, Inferior thyroid veins Superior paraesophageal nodes Accessory azygous vein Hemiazygous vein Paratracheal nodes Pulmonary hilar nodes Azygous vein Subcarinal nodes Inferior paraesophageal nodes Parahiatal nodes Short gastric veins Coronary vein Portal vein Superior mesenteric vein Internal jugular nodes Splenic artery nodes Left gastric artery nodes Hepatic artery nodes Celiac artery nodes Splenic vein Figure 25-9. Venous drainage of the esophagus. (Reproduced with permission from Shields TW: General Thoracic Surgery, 3rd ed. Philadelphia, PA: Lea & Febiger; 1989.) Figure 25-11. Lymphatic drainage of the esophagus. (Reproduced with permission from DeMeester TR, Barlow AP. Surgery and current management for cancer of the esophagus and cardia: Part I, Curr Probl Surg. 1988 Jul;25(7):475-531.) The act of alimentation requires the passage of food and drink from the mouth into the stomach. One-third of this distance consists of the mouth and hypopharynx, and two-thirds is made up by the esophagus. To comprehend the mechanics of alimentation, it is useful to visualize the gullet as a mechanical model in which the tongue and pharynx function as a piston pump with three valves, and the body of the esophagus and cardia function as a worm-drive pump with a single valve. The three valves in the pharyngeal cylinder are the soft palate, epiglottis, and cricopharyngeus. The valve of the esophageal pump is the LES. Failure of the valves or the pumps leads to abnormalities in swallowing—that is, difficulty in food propulsion from mouth to stomach—or regurgitation of gastric contents into the esophagus or pharynx. Food is taken into the mouth in a variety of bite sizes, where it is broken up, mixed with saliva, and lubricated. Once initiated, swallowing is entirely a reflex act. When food is ready for swallowing, the tongue, acting like a piston, moves the bolus into the posterior oropharynx and forces it into the hypopharynx (Fig. 25-12). Concomitantly with the posterior movement of the tongue, the soft palate is elevated, thereby closing the passage between the oropharynx and nasopharynx. This partitioning prevents pressure generated in the oropharynx from being dissipated through the nose. When the soft palate is paralyzed, for example, after a cerebrovascular accident, food is commonly regurgitated into the nasopharynx. During swallowing, the hyoid bone moves upward and anteriorly, elevating the larynx and opening the retrolaryngeal space, bringing the epiglottis under the tongue (see Fig. 25-12). The backward tilt of the epiglottis covers the opening of the larynx to prevent aspiration. The entire pharyngeal part of swallowing occurs within 1.5 seconds. During swallowing, the pressure in the hypopharynx rises abruptly, to at least 60 mmHg, due to the backward movement of the tongue and contraction of the posterior pharyngeal constrictors. A sizable pressure difference develops between the hypopharyngeal pressure and the less-than-atmospheric midesophageal or intrathoracic pressure (Fig. 25-13). This pressure 1 3 2 4 6 5 1. Elevation of tongue 2. Posterior movement of tongue 3. Elevation of soft palate 4. Elevation of hyoid 5. Elevation of larynx 6. Tilting of epiglottis Figure 25-12. Sequence of events during the oropharyngeal phase of swallowing. (Reproduced with permission from Zuidema GD, Orringer MB: Shackelford’s Surgery of the Alimentary Tract, 3rd ed. Vol 1. Philadelphia, PA: Elsevier/Saunders; 1991.) gradient speeds the movement of food from the hypopharynx into the esophagus when the cricopharyngeus or upper esophageal sphincter relaxes. The bolus is both propelled by peristaltic contraction of the posterior pharyngeal constrictors and sucked into the thoracic esophagus. Critical to receiving the bolus is the compliance of the cervical esophagus; when compliance is lost due to muscle pathology, dysphagia can result. The upper esophageal sphincter closes within 0.5 seconds of the initiation of the swallow, with the immediate closing pressure reaching P C E DES G 0 % Esophagus length PHYSIOLOGY Swallowing Mechanism 1015 20 Upright position 40 60 Air 80 100 –10 –5 0 5 10 15 20 25 30 35 40 Pressure (mm Hg) Figure 25-13. Resting pressure profile of the foregut showing the pressure differential between the atmospheric pharyngeal pressure (P) and the less-than-atmospheric midesophageal pressure (E) and greater-than-atmospheric intragastric pressure (G), with the interposed high-pressure zones of the cricopharyngeus (C) and distal esophageal sphincter (DES). The necessity for relaxation of the cricopharyngeus and DES pressure to move a bolus into the stomach is apparent. Esophageal work occurs when a bolus is pushed from the midesophageal area (E), with a pressure less than atmospheric, into the stomach, which has a pressure greater than atmospheric (G). (Reproduced with permission from Waters PF, DeMeester TR: Foregut motor disorders and their surgical managemen, Med Clin North Am. 1981 Nov;65(6):1235-1268.) CHAPTER 25 ESOPHAGUS AND DIAPHRAGMATIC HERNIA the submucosal lymph plexus extends over a long distance in a longitudinal direction before penetrating the muscle layer to enter lymph vessels in the adventitia. As a consequence of this nonsegmental lymph drainage, a primary tumor can extend for a considerable length superiorly or inferiorly in the submucosal plexus. Consequently, free tumor cells can follow the submucosal lymphatic plexus in either direction for a long distance before they pass through the muscularis and on into the regional LNs. The cervical esophagus has more direct segmental lymph drainage into the regional nodes, and, as a result, lesions in this portion of the esophagus have less submucosal extension and a more regionalized lymphatic spread. The efferent lymphatics from the cervical esophagus drain into the paratracheal and deep cervical LNs, and those from the upper thoracic esophagus empty mainly into the paratracheal LNs. Efferent lymphatics from the lower thoracic esophagus drain into the subcarinal nodes and nodes in the inferior pulmonary ligaments. The superior gastric nodes receive lymph not only from the abdominal portion of the esophagus, but also from the adjacent lower thoracic segment. 1016 60 50 40 30 20 10 0 Pharynx Cricopharyngeus 50 40 30 20 10 0 PART II 50 40 30 20 10 0 Esophageal body SPECIFIC CONSIDERATIONS 50 40 30 20 10 0 High pressure zone Stomach 50 40 30 20 10 0 mm Swallow Hg Seconds Seconds Seconds Seconds Seconds Figure 25-14. Intraluminal esophageal pressures in response to swallowing. (Reproduced with permission from Waters PF, DeMeester TR: Foregut motor disorders and their surgical managemen, Med Clin North Am. 1981 Nov;65(6):1235-1268.) approximately twice the resting level of 30 mmHg. The postrelaxation contraction continues down the esophagus as a peristaltic wave (Fig. 25-14). The high closing pressure and the initiation of the peristaltic wave prevents reflux of the bolus from the esophagus back into the pharynx. After the peristaltic wave has passed farther down the esophagus, the pressure in the upper esophageal sphincter returns to its resting level. Swallowing can be started at will, or it can be reflexively elicited by the stimulation of areas in the mouth and pharynx, among them the anterior and posterior tonsillar pillars or the posterior lateral walls of the hypopharynx. The afferent sensory nerves of the pharynx are the glossopharyngeal nerves and the superior laryngeal branches of the vagus nerves. Once aroused by stimuli entering via these nerves, the swallowing center in the medulla coordinates the complete act of swallowing by discharging impulses through cranial nerves V, VII, X, XI, and XII, as well as the motor neurons of C1 to C3. Discharges through these nerves occur in a rather specific pattern and last for approximately 0.5 seconds. Little is known about the organization of the swallowing center, except that it can trigger swallowing after a variety of different inputs, but the response is always a rigidly ordered pattern of outflow. Following a cerebrovascular accident, this coordinated outflow may be altered, causing mild to severe abnormalities of swallowing. In more severe injury, swallowing can be grossly disrupted, leading to repetitive aspiration. The striated muscles of the cricopharyngeus and the upper one-third of the esophagus are activated by efferent motor fibers distributed through the vagus nerve and its recurrent laryngeal branches. The integrity of innervation is required for the cricopharyngeus to relax in coordination with the pharyngeal contraction, and resume its resting tone once a bolus has entered the upper esophagus. Operative damage to the innervation can interfere with laryngeal, cricopharyngeal, and upper esophageal function, and predispose the patient to aspiration. The pharyngeal activity in swallowing initiates the esophageal phase. The body of the esophagus functions as a wormdrive propulsive pump due to the helical arrangement of its circular muscles, and it is responsible for transferring a bolus of food into the stomach. The esophageal phases of swallowing represent esophageal work done during alimentation, in that food is moved into the stomach from a negative-pressure environment of –6 mmHg intrathoracic pressure, to a positivepressure environment of 6 mmHg intra-abdominal pressure, or over a gradient of 12 mmHg (see Fig. 25-13). Effective and coordinated smooth muscle function in the lower one-third of the esophagus is therefore important in pumping the food across this gradient. The peristaltic wave generates an occlusive pressure varying from 30 to 120 mmHg (see Fig. 25-14). The wave rises to a peak in 1 second, lasts at the peak for about 0.5 seconds, and then subsides in about 1.5 seconds. The whole course of the rise and fall of occlusive pressure may occupy one point in the esophagus for 3 to 5 seconds. The peak of a primary peristaltic contraction initiated by a swallow (primary peristalsis) moves down the esophagus at 2 to 4 cm/s and reaches the distal esophagus about 9 seconds after swallowing starts. Consecutive swallows produce similar primary peristaltic waves, but when the act of swallowing is rapidly repeated, the esophagus remains relaxed and the peristaltic wave occurs only after the last movement of the pharynx. Progress of the wave in the esophagus is caused by sequential activation of its muscles, initiated by efferent vagal nerve fibers arising in the swallowing center. Continuity of the esophageal muscle is not necessary for sequential activation if the nerves are intact. If the muscles, but not the nerves, are cut across, the pressure wave begins distally below the cut as it dies out at the proximal end above the cut. This allows a sleeve resection of the esophagus to be done without destroying its normal function. Afferent impulses from receptors within the esophageal wall are not essential for progress of the coordinated wave. Afferent nerves, however, do go to the swallowing center from the esophagus because if the esophagus is distended at any point, a contraction wave begins with a forceful closure of the upper esophageal sphincter and sweeps down the esophagus. This secondary contraction occurs without any movements of the mouth or pharynx. Secondary peristalsis can occur as an independent local reflex to clear the esophagus of ingested material left behind after the passage of the primary wave. Current studies suggest that secondary peristalsis is not as common as once thought. Despite the powerful occlusive pressure, the propulsive force of the esophagus is relatively feeble. If a subject attempts to swallow a bolus attached by a string to a counterweight, the maximum weight that can be overcome is 5 to 10 g. Orderly contractions of the muscular wall and anchoring of the esophagus at its inferior end are necessary for efficient aboral propulsion to occur. Loss of the inferior anchor, as occurs with a large hiatal hernia, can lead to inefficient propulsion. The LES provides a pressure barrier between the esophagus and stomach and acts as the valve on the worm-drive pump of the esophageal body. Although an anatomically distinct LES has been difficult to identify, microdissection studies show that, in humans, the sphincter-like function is related to the -50 50- 0- Phrenoesophageal membrane Semi-circular fibers Gastro-esophageal muscular ring -0 mm Oblique fibers -50 -0 mm -20 Anterior wall thickness Figure 25-15. Wall thickness and orientation of fibers on microdissection of the cardia. At the junction of the esophageal tube and gastric pouch, there is an oblique muscular ring composed of an increased muscle mass inside the inner muscular layer. On the lesser curve side of the cardia, the muscle fibers of the inner layer are oriented transversely and form semicircular muscle clasps. On the greater curve side of the cardia, these muscle fibers form oblique loops that encircle the distal end of the cardia and gastric fundus. Both the semicircular muscle clasps and the oblique fibers of the fundus contract in a circular manner to close the cardia. (Reproduced with permission from Glenn WWL: Thoracic and Cardiovascular Surgery, 4th ed. Norwalk, CT: Appleton-Century-Crofts; 1983.) architecture of the muscle fibers at the junction of the esophageal tube with the gastric pouch (Fig. 25-15). The sphincter actively remains closed to prevent reflux of gastric contents into the esophagus and opens by a relaxation that coincides with a pharyngeal swallow (see Fig. 25-14). The LES pressure returns to its resting level after the peristaltic wave has passed through the esophagus. Consequently, reflux of gastric juice that may occur through the open valve during a swallow is cleared back into the stomach. If the pharyngeal swallow does not initiate a peristaltic contraction, then the coincident relaxation of the LES is unguarded and reflux of gastric juice can occur. This may be an explanation for the observation of spontaneous lower esophageal relaxation, thought by some to be a causative factor in gastroesophageal reflux disease (GERD). The power of the worm-drive pump of the esophageal body is insufficient to force open a valve that does not relax. In dogs, a bilateral cervical parasympathetic blockade abolishes the relaxation of the LES that occurs with pharyngeal swallowing or distention of the esophagus. Consequently, vagal function appears to be important in coordinating the relaxation of the LES with esophageal contraction. The antireflux mechanism in human beings is composed of three components: a mechanically effective LES, efficient esophageal clearance, and an adequately functioning gastric reservoir. A defect of any one of these three components can lead to increased esophageal exposure to gastric juice and the development of mucosal injury. On 24-hour esophageal pH monitoring, healthy individuals have occasional episodes of gastroesophageal reflux. This physiologic reflux is more common when awake and in the upright position than during sleep in the supine position. When reflux of gastric juice occurs, normal subjects rapidly clear the acid gastric juice from the esophagus regardless of their position. There are several explanations for the observation that physiologic reflux in normal subjects is more common when they are awake and in the upright position than during sleep in the supine position. First, reflux episodes occur in healthy volunteers primarily during transient losses of the gastroesophageal barrier, which may be due to a relaxation of the LES or intragastric pressure overcoming sphincter pressure. Gastric juice can also reflux when a swallow-induced relaxation of the LES is not protected by an oncoming peristaltic wave. The average frequency of these “unguarded moments” or of transient losses of the gastroesophageal barrier is far less while asleep and in the supine position than while awake and in the upright position. Consequently, there are fewer opportunities for reflux to occur in the supine position. Second, in the upright position, there is a 12-mmHg pressure gradient between the resting, positive intra-abdominal pressure measured in the stomach and the most negative intrathoracic pressure measured in the esophagus at midthoracic level. This gradient favors the flow of gastric juice up into the thoracic esophagus when upright. The gradient diminishes in the supine position. Third, the LES pressure in normal subjects is significantly higher in the supine position than in the upright position. This is due to the apposition of the hydrostatic pressure of the abdomen to the abdominal portion of the sphincter when supine. In the upright position, the abdominal pressure surrounding the sphincter is negative compared with atmospheric pressure, and, as expected, the abdominal pressure gradually increases the more caudally it is measured. This pressure gradient tends to move the gastric contents toward the cardia and encourages the occurrence of reflux into the esophagus when the individual is upright. In contrast, in the supine position, the gastroesophageal pressure gradient diminishes, and the abdominal hydrostatic pressure under the diaphragm increases, causing an increase in sphincter pressure and a more competent cardia. The LES has intrinsic myogenic tone, which is modulated by neural and hormonal mechanisms. α-Adrenergic neurotransmitters or β-blockers stimulate the LES, and α-blockers and β-stimulants decrease its pressure. It is not clear to what extent cholinergic nerve activity controls LES pressure. The vagus nerve carries both excitatory and inhibitory fibers to the esophagus and sphincter. The hormones gastrin and motilin have been shown to increase LES pressure; and cholecystokinin, estrogen, glucagon, progesterone, somatostatin, and secretin decrease LES pressure. The peptides bombesin, l-enkephalin, and substance P increase LES pressure; and calcitonin generelated peptide, gastric inhibitory peptide, neuropeptide Y, and vasoactive intestinal polypeptide decrease LES pressure. Some pharmacologic agents such as antacids, cholinergics, agonists, domperidone, metoclopramide, and prostaglandin F2 are known to increase LES pressure; and anticholinergics, barbiturates, calcium channel blockers, caffeine, diazepam, dopamine, meperidine, prostaglandin E1 and E2, and theophylline decrease LES pressure. Peppermint, chocolate, coffee, ethanol, and fat are all associated with decreased LES pressure and may be responsible for esophageal symptoms after a sumptuous meal. 1017 CHAPTER 25 ESOPHAGUS AND DIAPHRAGMATIC HERNIA Greater curvature wall thickness 20- Lesser curvature wall thickness -20 Physiologic Reflux 1018 ASSESSMENT OF ESOPHAGEAL FUNCTION A thorough understanding of the patient’s underlying anatomic and functional deficits before making therapeutic decisions is fundamental to the successful treatment of esophageal disease. The diagnostic tests, as presently used, may be divided into four groups: (a) tests to detect structural abnormalities of 1 broad the esophagus; (b) tests to detect functional abnormalities of the esophagus; (c) tests to detect increased esophageal exposure to gastric juice; and (d) tests of duodenogastric function as they relate to esophageal disease. PART II Tests to Detect Structural Abnormalities SPECIFIC CONSIDERATIONS Endoscopic Evaluation. The first diagnostic test in patients with suspected esophageal disease is usually upper gastrointestinal endoscopy. This allows assessment and biopsy of the mucosa of the stomach and the esophagus, as well as the diagnosis and assessment of obstructing lesions in the upper gastrointestinal tract. In any patient complaining of dysphagia, esophagoscopy is indicated, even in the face of a normal radiographic study. For the initial endoscopic assessment, the flexible fiberoptic esophagoscope is the instrument of choice because of its technical ease, patient acceptance, and the ability to simultaneously assess the stomach and duodenum. Rigid endoscopy is now only rarely required, mainly for the disimpaction of difficult foreign bodies impacted in the esophagus, and few individuals now have the skill set and experience to use this equipment. When GERD is the suspected diagnosis, particular attention should be paid to detecting the presence of esophagitis and Barrett’s columnar-lined esophagus (CLE). When endoscopic esophagitis is seen, severity and the length of esophagitis involved are recorded. Whilst many different grading systems have been proposed, the commonest system now in use is the Los Angeles (LA) grading system. In this system, mild esophagitis is classified LA grade A or B—one or more erosions limited to the mucosal fold(s) and either less than or greater than 5 mm in longitudinal extent respectively (Fig. 25-16). More severe esophagitis is classified LA grade C or D. In grade C, erosions extend over the mucosal folds but over less than threequarters of the esophageal circumference; in grade D, confluent erosions extend across more than three-quarters of the esophageal circumference. In addition to these grades, more severe damage can lead to the formation of a stricture. A stricture’s severity can be assessed by the ease of passing a standard endoscope. When a stricture is observed, the severity of the esophagitis above it should be recorded. The absence of esophagitis above a stricture suggests the possibility of a chemical-induced injury or a neoplasm as a cause. The latter should always be considered and is ruled out only by evaluation of a tissue biopsy of adequate size. It should be remembered that gastroesophageal reflux is not always associated with visible mucosal abnormalities, and patients can experience significant reflux symptoms, despite an apparently normal endoscopy examination. Barrett’s esophagus (BE) is a condition in which the tubular esophagus is lined with columnar epithelium, as opposed to the normal squamous epithelium (see Fig. 25-16). Histologically, it appears as intestinal metaplasia (IM). It is suspected at endoscopy when there is difficulty in visualizing the squamocolumnar junction at its normal location, and by the appearance of a redder, salmon-colored mucosa in the lower esophagus, with a clearly visible line of demarcation at the top of the Barrett’s esophagus segment. Its presence is confirmed by biopsy. Multiple biopsy specimens should be taken in a cephalad direction to confirm the presence of IM, and to evaluate the Barrett’s epithelium for dysplastic changes. BE is susceptible to ulceration, bleeding, stricture formation, and, most important, malignant degeneration. The earliest sign of the latter is high grade dysplasia or intramucosal adenocarcinoma (see Fig. 25-16). These dysplastic changes have a patchy distribution, so a minimum of four biopsy samples spaced 2 cm apart should be taken from the Barrett’s-lined portion of the esophagus. Changes seen in one biopsy are significant. Nishimaki has determined that the tumors occur in an area of specialized columnar epithelium near the squamocolumnar junction in 85% of patients, and within 2 cm of the squamocolumnar junction in virtually all patients. Particular attention should be focused on this area in patients suspected of harboring a carcinoma. Abnormalities of the gastroesophageal flap valve can be visualized by retroflexion of the endoscope. Hill has graded the appearance of the gastroesophageal valve from I to IV according to the degree of unfolding or deterioration of the normal valve architecture (Fig. 25-17). The appearance of the valve correlates with the presence of increased esophageal acid exposure, occurring predominantly in patients with grade III and IV valves. A hiatal hernia is endoscopically confirmed by finding a pouch lined with gastric rugal folds lying 2 cm or more above the margins of the diaphragmatic crura, identified by having the patient sniff. A hernia is best demonstrated with the stomach fully insufflated and the gastroesophageal junction observed with a retroflexed endoscope. A prominent sliding hiatal hernia frequently is associated with increased esophageal exposure to gastric juice. When a paraesophageal hernia (PEH) is observed, particular attention is taken to exclude gastric (Cameron’s) ulcers or gastritis within the pouch. The intragastric retroflex or J maneuver is important in evaluating the full circumference of the mucosal lining of the herniated stomach. When an esophageal diverticulum is seen, it should be carefully explored with the flexible endoscope to exclude ulceration or neoplasia. When a submucosal mass is identified, biopsy specimens are usually not performed. At the time of surgical resection, a submucosal leiomyoma or reduplication cyst can generally be dissected away from the intact mucosa, but if a biopsy sample is taken, the mucosa may become fixed to the underlying abnormality. This complicates the surgical dissection by increasing the risk of mucosal perforation. Endoscopic ultrasound provides a better method for evaluating these lesions. Radiographic Evaluation. Barium swallow evaluation is undertaken selectively to assess anatomy and motility. The anatomy of large hiatal hernias is more clearly demonstrated by contrast radiology than endoscopy, and the presence of coordinated esophageal peristalsis can be determined by observing several individual swallows of barium traversing the entire length of the organ, with the patient in the horizontal position. Hiatal hernias are best demonstrated with the patient prone because the increased intraabdominal pressure produced in this position promotes displacement of the esophagogastric junction above the diaphragm. To detect lower esophageal narrowing, such as rings and strictures, fully distended views of the esophagogastric region are crucial. The density of the barium used to study the esophagus can potentially affect the accuracy of the examination. Esophageal disorders shown clearly by a full-column technique include circumferential carcinomas, peptic strictures, large esophageal ulcers, and hiatal hernias. A small hiatal hernia is usually not associated with significant symptoms or illness, and its presence is an irrelevant finding unless the hiatal hernia is large (Fig. 25-18) or the hernia 1019 B C D Figure 25-16. Complications of reflux disease as seen on endoscopy. A. Linear erosions of LA grade B esophagitis. B. Uncomplicated Barrett’s mucosa. C. High-grade dysplasia in Barrett’s mucosa. D. Early adenocarcinoma arising in Barrett’s mucosa. is of the paraesophageal variety. Lesions extrinsic but adjacent to the esophagus can be reliably detected by the full-column technique if they contact the distended esophageal wall. Conversely, a number of important disorders may go undetected if this is the sole technique used to examine the esophagus. These include small esophageal neoplasms, mild esophagitis, and esophageal varices. Thus, the full-column technique should be supplemented with mucosal relief or double-contrast films to enhance detection of these smaller or more subtle lesions. Motion-recording techniques greatly aid in evaluating functional disorders of the pharyngoesophageal and esophageal phases of swallowing. The technique and indications for cineand videoradiography will be discussed in the section entitled “Video- and Cineradiography,” as they are more useful to evaluate function and seldom used to detect structural abnormalities. The radiographic assessment of the esophagus is not complete unless the entire stomach and duodenum have been examined. A gastric or duodenal ulcer, partially obstructing gastric neoplasm, or scarred duodenum and pylorus may contribute significantly to symptoms otherwise attributable to an esophageal abnormality. When a patient’s complaints include dysphagia and no obstructing lesion is seen on the barium swallow, it is useful to have the patient swallow a barium-impregnated marshmallow, a barium-soaked piece of bread, or a hamburger mixed with barium. This test may bring out a functional disturbance in esophageal transport that can be missed when liquid barium is used. Tests to Detect Functional Abnormalities In many patients with symptoms of an esophageal disorder, standard radiographic and endoscopic evaluation fails to demonstrate a structural abnormality. In these situations, esophageal function tests are necessary to identify a functional disorder. Esophageal Motility. Esophageal motility is a widely used technique to examine the motor function of the esophagus and CHAPTER 25 ESOPHAGUS AND DIAPHRAGMATIC HERNIA A 1020 PART II SPECIFIC CONSIDERATIONS A B C Figure 25-17. A. Grade I flap valve appearance. Note the ridge of tissue that is closely approximated to the shaft of the retroflexed endoscope. It extends 3 to 4 cm along the lesser curve. B. Grade II flap valve appearance. The ridge is slightly less well defined than in grade I and it opens rarely with respiration and closes promptly. C. Grade III flap valve appearance. The ridge is barely present, and there is often failure to close around the endoscope. It is nearly always accompanied by a hiatal hernia. D. Grade IV flap valve appearance. There is no muscular ridge at all. The gastroesophageal valve stays open all the time, and squamous epithelium can often be seen from the retroflexed position. A hiatal hernia is always present. (Reproduced with permission from Hill LD, Kozarek RA, Kraemer SJ, et al: The gastroesophageal flap valve: in vitro and in vivo observations, Gastrointest Endosc. 1996 Nov;44(5):541-547.) 1021 its sphincters. The esophageal motility study (EMS) is indicated whenever a motor abnormality of the esophagus is suspected on the basis of complaints of dysphagia, odynophagia, or noncardiac chest pain, and the barium swallow or endoscopy does not show a clear structural abnormality. EMS is particularly necessary to confirm the diagnosis of specific primary esophageal motility disorders (i.e., achalasia, diffuse esophageal spasm [DES], nutcracker esophagus, and hypertensive LES). It also identifies nonspecific esophageal motility abnormalities and motility disorders secondary to systemic disease such as scleroderma, dermatomyositis, polymyositis, or mixed connective tissue disease. In patients with symptomatic GERD, manometry of the esophageal body can identify a mechanically defective LES and evaluate the adequacy of esophageal peristalsis and contraction amplitude. EMS has become an essential tool in the preoperative evaluation of patients before antireflux surgery, guiding selection of the appropriate procedure based upon the patient’s underlying esophageal function and excluding patients with achalasia who can be misdiagnosed with gastroesophageal reflux when clinical and endoscopic parameters alone are used for diagnosis. EMS is performed using electronic, pressure-sensitive transducers located within the catheter, or water-perfused catheters with lateral side holes attached to transducers outside the body. The traditional water perfused catheter has largely been replaced by high resolution motility (HRM), but knowledge of traditional methods of assessing esophageal motility is helpful for understanding esophageal physiology. As the pressure-sensitive station is brought across the gastroesophageal junction (GEJ), a rise in pressure above the gastric baseline signals the beginning of the LES. The respiratory inversion point is identified when the positive excursions that occur in the abdominal cavity with breathing change to negative deflections in the thorax. The respiratory inversion point serves as a reference point at which the amplitude of LES pressure and the length of the sphincter exposed to abdominal pressure are measured. As the pressure-sensitive station is withdrawn into the body of the esophagus, the upper border of the LES is identified by the drop in pressure to the esophageal baseline. From these measurements, the pressure, abdominal length, and overall length of the sphincter are determined (Fig. 25-19). To Overall length Gastric baseline pressure 43 Pressure 42 41 40 10 sec Abdominal length 39 RIP 38 37 cm Esophageal baseline pressure RIP = Respiratory inversion point Figure 25-18. Radiogram of an intrathoracic stomach. This is the end stage of a large hiatal hernia, regardless of its initial classification. Figure 25-19. Manometric pressure profile of the lower esophageal sphincter. The distances are measured from the nares. (Reproduced with permission from Zaninotto G, DeMeester TR, Schwizer W, et al: The lower esophageal sphincter in health and disease, Am J Surg. 1988 Jan;155(1):104-11.) CHAPTER 25 ESOPHAGUS AND DIAPHRAGMATIC HERNIA D Figure 25-17. (Continued ) 1022 foregut disorder. A mechanically defective sphincter is identified by having one or more of the following characteristics: an average LES pressure of <6 mmHg, an average length exposed to the positive-pressure environment in the abdomen of 1 cm or less, and/or an average overall sphincter length of 2 cm or less. LP P 0 25 RP L 50 PART II LA R SPECIFIC CONSIDERATIONS RA A Figure 25-20. Radial configuration of the lower esophageal sphincter. A = anterior; L = left; LA = left anterior; LP = left posterior; P = posterior; R = right; RA = right anterior; RP = right posterior. (Reproduced with permission from Winans CS: Manometric asymmetry of the lower-esophageal high-pressure zone, Am J Dig Dis. 1977 Apr;22(4):348-354.) account for the asymmetry of the sphincter (Fig. 25-20), the pressure profile is repeated with each of the five radially oriented transducers, and the average values for sphincter pressure above gastric baseline, overall sphincter length, and abdominal length of the sphincter are calculated. Table 25-1 shows the values for these parameters in 50 normal volunteers without subjective or objective evidence of a Table 25-1 Normal manometric values of the distal esophageal sphincter, n = 50 PERCENTILE MEDIAN 2.5 97.5 13 5.8 27.7 Overall length (cm) 3.6 2.1 5.6 Abdominal length (cm) 2 0.9 4.7 MEAN MEAN – 2 SD MEAN + 2 SD Pressure (mmHg) Pressure (mmHg) 13.8 ± 4.6 4.6 23.0 Overall length (cm) 3.7 ± 0.8 2.1 5.3 Abdominal length (cm) 0.6 3.8 2.2 ± 0.8 SD = standard deviation. Reproduced with permission from Moody FG, Carey LC, Jones RS, et al: Surgical Treatment of Digestive Disease. Chicago, IL: Year Book Medical; 1990. High-Resolution Manometry. Esophageal manometry was introduced into clinical practice in the 1970s and, until recently, has changed little. In 1991, Ray Clouse introduced the concept of improving conventional manometry by increasing the number of recording sites and adding a three-dimensional assessment. This “high-resolution manometry” is a variant of the conventional manometry in which multiple, circumferential recording sites are used, in essence creating a “map” of the esophagus and its sphincters. High-resolution catheters contain 36 miniaturized pressure sensors positioned every centimeter along the length of the catheter. The vast amount of data generated by these sensors is then processed and presented in traditional linear plots or as a visually enhanced spatiotemporal video tracing that is readily interpreted. The function of the esophageal body is assessed with 10 to 15 wet swallows. Amplitude, duration, and morphology of contractions following each swallow are visually displayed (Fig. 25-21). The relationship of the esophageal contractions following a swallow is classified as peristaltic or simultaneous. The data are used to identify motor disorders of the esophagus. The position, length, and function of the lower esophageal sphincter (LES) are demonstrated by a high-pressure zone that should relax at the inception of swallowing and contract after the water or solid bolus passes through the LES. Simultaneous acquisition of data for the upper esophageal sphincter, esophageal body, LES, and gastric pressure minimizes the movement artifacts and study time associated with conventional esophageal manometry. This technology significantly enhances esophageal diagnostics, bringing it into the realm of “image”-based studies. High-resolution manometry may allow the identification of focal motor abnormalities previously overlooked. It has enhanced the ability to predict bolus propagation and increased sensitivity in the measurement of pressure gradients. Esophageal Impedance. Newer technology introduced into the clinical realm a decade ago allows measurement of esophageal function and gastroesophageal reflux in a way that was previously not possible. An intraluminal electrical impedance catheter is used to measure GI function. Impedance is the ratio of voltage to current, and is a measure of the electrical conductivity of a hollow organ and its contents. Intraluminal electrical impedance is inversely proportional to the electrical conductivity of the luminal contents and the cross-sectional area of the lumen. Air has a very low electrical conductivity and, therefore, high impedance. Saliva and food cause an impedance decrease because of their increased conductivity. Luminal dilatation results in a decrease in impedance, whereas luminal contraction yields an impedance increase. Investigators have established the impedance waveform characteristics that define esophageal bolus transport. This allows for the characterization of both esophageal function, via quantification of bolus transport, and gastroesophageal reflux (Fig. 25-22). The probe measures impedance between adjacent electrodes, with measuring segments located at 2, 4, 6, 8, 14, and 16 cm from the distal tip. An extremely low electric current of 0.00025 μW is transmitted across the electrodes at a frequency of 1 to 2 kHz and is limited Pharynx Esophagus 40.3 43.7 PIP 42.3 Gastric 46.2 Stomac 1A. Normal high-resolution manometry motility study. Pressure measurements are recorded with color coding (red = high; blue = low). LES = lower esophageal sphincter; P int; UES = upper esophageal sphincter. CHAPTER 25 ESOPHAGUS AND DIAPHRAGMATIC HERNIA PART II Pharynx Esophagus 41.3 PIP 42.7 41.8 Stomach Gastric 50.3 SPECIFIC CONSIDERATIONS 1B. High-resolution manometry motility study in patient with mechanically defective lower esophageal sphincter. Note the absence of lower esophageal sphincter tone. Press corded with color coding (red = high; blue = low). LES = lower esophageal sphincter; PIP = pressure inversion point; UES = upper esophageal sphincter. Pharynx Esophagus 40.9 PIP 42.3 44.6 Gastric 47.5 Stomach 1C. High-resolution manometry motility study in patient with deficient esophageal body peristalsis. Note the very weak peristalsis in the lower two-thirds of the esopha ts are recorded with color coding (red = high; blue = low). LES = lower esophageal sphincter; PIP = pressure inversion point; UES = upper esophageal sphincter. CHAPTER 25 ESOPHAGUS AND DIAPHRAGMATIC HERNIA PART II Pharynx Esophagus 42.7 45.7 PIP 44.1 Stomach Gastric 48.5 SPECIFIC CONSIDERATIONS 1D. High-resolution manometry motility study in patient with achalasia. Note the complete absence of esophageal body peristalsis, and the lack of relaxation of the lowe essure measurements are recorded with color coding (red = high; blue = low). LES = lower esophageal sphincter; PIP = pressure inversion point; UES = upper esophageal Pharynx Esophagus 45.6 PIP 47.1 49.7 Stoma Gastric 51.7 1E. High-resolution manometry motility study in patient with diffuse esophageal spasm. Note the very high amplitude contractions in the esophageal body. Pressure meas h color coding (red = high; blue = low). LES = lower esophageal sphincter; PIP = pressure inversion point; UES = upper esophageal sphincter. CHAPTER 25 ESOPHAGUS AND DIAPHRAGMATIC HERNIA 1028 Impedence site pH site 17cm Distance above LES PART II Distance above LES 15cm 9cm 7cm 5cm 5cm 3cm SPECIFIC CONSIDERATIONS LES Figure 25-22. Esophageal impedance probe measures electrical resistance between evenly spaced electrodes. LES = lower esophageal sphincter. to 8 μA. This is below the stimulation threshold for nerves and muscles and is three orders of magnitude below the threshold of cardiac stimulation. A standard pH electrode is located 5 cm from the distal tip so that the acidic or nonacidic nature of refluxate can be correlated with the number of reflux events. Esophageal impedance has been validated as an appropriate method for the evaluation of GI function and is used selectively for the diagnosis of gastroesophageal reflux. It has been compared to cineradiography showing that impedance waves correspond well with actual bolus transport illustrated by radiography. Bolus entry, transit, and exit can be clearly identified by impedance changes in the corresponding measuring segments. Studies comparing standard esophageal manometry with impedance measurements in healthy volunteers have shown that esophageal impedance correlates with peristaltic wave progression and bolus length. Twenty-four-hour pH monitoring, the historical gold standard for diagnosing and quantifying gastroesophageal reflux, has some significant limitations. With 24-hour ambulatory pH testing, reflux is defined as a drop in the pH below 4, which effectively “blinds” the test to reflux occurring at higher pH values. Furthermore, in patients with persistent symptoms on proton pump inhibitor (PPI) therapy, pH monitoring has limited use as it can only detect abnormal acid reflux (pH <4), the occurrence of which has been altered by the antisecretory medication. Given that PPI antisecretory therapy is highly effective in neutralizing gastric acid, the question of whether persistent symptoms are a result of persistent acid reflux, nonacid reflux, or are not reflux related becomes a key issue in surgical decision making. Until recently, this differentiation could not be made. Detection of both acid and nonacid reflux has potential to define these populations of patients and thus improve patient selection for antireflux surgery. Multichannel intraluminal impedance technology allows the measurement of both acid and nonacid reflux, with potential to enhance diagnostic accuracy. Using this technology, Balaji and colleagues showed that most gastroesophageal reflux remains despite acid suppression. Impedance pH may be particularly useful in evaluating patients with persistent symptoms despite PPI treatment, patients with respiratory symptoms, and postoperative patients who are having symptoms that are elusive to diagnosis. Esophageal Transit Scintigraphy. The esophageal transit of a 10-mL water bolus containing technetium-99m (99mTc) sulfur colloid can be recorded with a gamma camera. Using this technique, delayed bolus transit has been shown in patients with a variety of esophageal motor disorders, including achalasia, scleroderma, DES, and nutcracker esophagus. Video- and Cineradiography High-speed cinematic or video recording of radiographic studies allows re-evaluation by reviewing the studies at various speeds. This technique is more useful than manometry in the evaluation of the pharyngeal phase of swallowing. Observations suggesting oropharyngeal or cricopharyngeal dysfunction include misdirection of barium into the trachea or nasopharynx, prominence of the cricopharyngeal muscle, a Zenker’s diverticulum, a narrow pharyngoesophageal segment, and stasis of the contrast medium in the valleculae or hypopharyngeal recesses (Fig. 25-23). These findings are usually not specific, but rather common manifestations of neuromuscular disorders affecting the pharyngoesophageal area. Studies using liquid barium, barium-impregnated solids, or radiopaque pills aid the evaluation of normal and abnormal motility in the esophageal body. Loss of the normal stripping wave or segmentation of the barium column with the patient in the recumbent position correlates with abnormal motility of the esophageal body. In addition, structural abnormalities such as small diverticula, webs, and minimal extrinsic impressions of the esophagus may be recognized only with motion-recording techniques. The simultaneous computerized capture of videofluoroscopic images and manometric tracings is now available and is referred to as manofluorography. Manofluorographic studies allow precise correlation of the anatomic events, such as opening of the upper esophageal sphincter, with manometric observations, such as sphincter relaxation. Manofluorography, although not widely available, is presently the best means available to evaluate complex functional abnormalities. Tests to Detect Increased Exposure to Gastric Juice Twenty-Four-Hour Ambulatory pH Monitoring. The most direct method of measuring increased esophageal exposure to gastric juice is by an indwelling pH electrode, or, more recently, via a radiotelemetric pH monitoring capsule that can be clipped to the esophageal mucosa. The latter consists of an antimony pH electrode fitted inside a small, capsule-shaped device accompanied by a battery and electronics that allow 48-hour monitoring and transmission of the pH data via transcutaneous radio telemetry to a waist-mounted data logger. The device can be introduced either transorally or transnasally, and it can be clipped to the esophageal mucosa using endoscopic fastening techniques. It passes spontaneously within 1 to 2 weeks. Prolonged monitoring of esophageal pH is performed by placing the pH probe or telemetry capsule 5 cm above the manometrically measured upper border of the distal sphincter for 24 hours. It measures the actual time the esophageal mucosa is exposed to gastric juice, measures the ability of the esophagus to clear refluxed acid, and correlates esophageal acid exposure with the patient’s symptoms. A 24- to 48-hour period is necessary so that measurements can be made over one or two complete circadian cycles. This allows measuring the effect of physiologic activity, such as eating or sleeping, on the reflux of gastric juice into the esophagus (Fig. 25-24). 1029 B The 24-hour esophageal pH monitoring should not be considered a test for reflux, but rather a measurement of the esophageal exposure to gastric juice. The measurement is expressed by the time the esophageal pH was below a given threshold during the 24-hour period (Table 25-3). This single assessment, although concise, does not reflect how the exposure has occurred; that is, did it occur in a few long episodes or several pH 8 mp mp 6 4 2 14:00 16:00 18:00 20:00 22:00 pH sp 8 short episodes? Consequently, two other assessments are necessary: the frequency of the reflux episodes and their duration. The units used to express esophageal exposure to gastric juice are: (a) cumulative time the esophageal pH is below a chosen threshold, expressed as the percentage of the total, upright, and supine monitored time; (b) frequency of reflux episodes below a chosen threshold, expressed as number of episodes per 24 hours; and (c) duration of the episodes, expressed as the number of episodes >5 minutes per 24 hours, and the time in minutes of the longest episode recorded. Table 25-2 shows the normal values for these components of the 24-hour record at the whole-number pH threshold derived from 50 normal asymptomatic subjects. The upper limits of normal were established at the 95th percentile. Most centers use pH 4 as the threshold. Based on these studies and extensive clinical experience, 48-hour esophageal pH monitoring is considered to be the gold standard for the diagnosis of GERD. The Bravo pH Capsule (Medtronics, Minneapolis, MN) measures pH levels in the esophagus and transmits continuous 6 4 2 Table 25-2 22:00 pH 8 00:00 02:00 04:00 06:00 Normal values for esophageal exposure to pH <4 (n = 50) COMPONENT MEAN SD 95% 6 Total time 1.51 1.36 4.45 4 Upright time 2.34 2.34 8.42 2 Supine time 0.63 1.0 3.45 No. of episodes 19.00 12.76 46.90 No. >5 min 0.84 1.18 3.45 Longest episode 6.74 7.85 19.80 06:00 mp 08:00 10:00 12:00 14:00 Figure 25-24. Strip chart display of a 24-hour esophageal pH monitoring study in a patient with increased esophageal acid exposure. mp = meal period; sp = supine period. (Reproduced with permission from Zuidema GD, Orringer MB: Shackelford’s Surgery of the Alimentary Tract, 3rd ed. Vol 1. Philadelphia, PA: Elsevier/ Saunders; 1991.) SD = standard deviation. Reproduced with permission from Moody FG, Carey LC, Jones RS, et al: Surgical Treatment of Digestive Disease. Chicago, IL: Year Book Medical; 1990. CHAPTER 25 ESOPHAGUS AND DIAPHRAGMATIC HERNIA A Figure 25-23. Esophagograms from a patient with cricopharyngeal achalasia. A. Anteroposterior film showing retention of the contrast medium at the level of the vallecula and piriform recesses, with no barium passing into the esophagus. B. Lateral film, taken opposite the C5–C6 vertebrae, showing posterior indentation of the cricopharyngeus, retention in the hypopharynx, and tracheal aspiration. (Reproduced with permission from DeMeester TR, Matthews H: International Trends in General Thoracic Surgery. Vol 3. Benign Esophageal Disease. St. Louis, Mo: Mosby; 1987.) 1030 Table 25-3 Normal composite score for various pH thresholds: upper level of normal value PART II pH THRESHOLD 95TH PERCENTILE <1 14.2 <2 17.37 <3 14.10 <4 14.72 <5 15.76 <6 12.76 >7 14.90 >8 8.50 SPECIFIC CONSIDERATIONS Reproduced with permission from Moody FG, Carey LC, Jones RS, et al: Surgical Treatment of Digestive Disease. Chicago, IL: Year Book Medical; 1990. esophageal pH readings to a receiver worn on the patient’s belt or waistband (Fig. 25-25). Symptoms that the patient experiences are recorded in a diary and/or by pressing buttons on the receiver unit. Generally, 48 hours of pH data are measured with this probe. A recent study has shown that the addition of a second day of pH monitoring increased the sensitivity of pH measurement by 22%. The capsule eventually detaches and passes through the digestive tract in 5 to 7 days. Radiographic Detection of Gastroesophageal Reflux. The definition of radiographic gastroesophageal reflux varies depending on whether reflux is spontaneous or induced by various maneuvers. In only about 40% of patients with classic symptoms of GERD is spontaneous reflux (i.e., reflux of barium from the stomach into the esophagus with the patient in the upright position) by the radiologist. In most patients who show spon2 observed taneous reflux on radiography, the diagnosis of increased esophageal acid exposure is confirmed by 24-hour esophageal pH monitoring. Therefore, the radiographic demonstration of spontaneous regurgitation of barium into the esophagus in the upright position is a reliable indicator that reflux is present. However, failure to see this does not indicate the absence of disease, and for this reason this test is rarely used for clinical diagnosis. Tests of Duodenogastric Function Esophageal disorders are frequently associated with abnormalities of duodenogastric function. Abnormalities of the gastric reservoir or increased gastric acid secretion can be responsible for increased esophageal exposure to gastric juice. Reflux of alkaline duodenal juice, including bile salts, pancreatic enzymes, and bicarbonate, is thought to have a role in the pathogenesis of esophagitis and complicated Barrett’s esophagus. Furthermore, functional disorders of the esophagus are often not confined to pH 7 4 2 10:00 pH probe 12:00 14:00 16:00 18:00 20:00 22:00 00:00 02:00 04:00 06:00 08:00 10:00 7 4 5 cm above 2 18:00 Combined 24-hour gastric and esophageal pH monitoring 5 cm below 7 4 2 02:00 A B Figure 25-25. A. Combined esophageal and gastric pH monitoring showing position of probes in relation to the lower esophageal sphincter. B. Combined ambulatory esophageal (upper tracing) and gastric (lower tracing) pH monitoring showing duodenogastric reflux (arrows) with propagation of the alkaline juice into the esophagus of a patient with complicated Barrett’s esophagus. The gastric tracing (lower) is taken from a probe lying 5 cm below the upper esophageal sphincter. The esophageal tracing (upper) is taken from a probe lying 5 cm above the lower esophageal sphincter. Note that in only a small proportion of time does duodenogastric reflux move the pH of the esophagus above the threshold of 7, causing the iceberg effect. (Reproduced with permission from Zuidema GD, Orringer MB: Shackelford’s Surgery of the Alimentary Tract, 3rd ed. Vol 1. Philadelphia, PA: Elsevier/Saunders; 1991.) the esophagus alone, but are associated with functional disorders of the rest of the foregut (i.e., stomach and duodenum). Tests of duodenogastric function that are helpful to investigate esophageal symptoms include gastric emptying studies, gastric acid analysis, and cholescintigraphy (for the diagnosis of pathologic duodenogastric and/or duodenogastroesophageal reflux). GASTROESOPHAGEAL REFLUX DISEASE GERD was not recognized as a significant clinical problem until the mid-1930s and was not identified as a precipitating cause for esophagitis until after World War II. In the early 21st century, it has grown to be a very common problem and now accounts for a majority of esophageal pathology. It is recognized as a chronic disease, and when medical therapy is required, it is often lifelong treatment. Recent efforts at the development of various endoscopic antireflux interventions, although innovative, have not been successful in consistently controlling gastroesophageal reflux. Antireflux surgery is an effective and long-term therapy and is the only treatment that is able to restore the gastroesophageal barrier. Despite the common prevalence of GERD, it can be one of the most challenging diagnostic and therapeutic problems in clinical medicine. A contributing factor to this is the lack of a universally accepted definition of the disease. The most simplistic approach is to define the disease by its symptoms. However, symptoms thought to be indicative of GERD, such as heartburn or acid regurgitation, are very common in the general population and many individuals consider them to be normal and do not seek medical attention. Even when excessive, these symptoms are not specific for gastroesophageal reflux. They can be caused by other diseases such as achalasia, DES, esophageal carcinoma, pyloric stenosis, cholelithiasis, gastritis, gastric or duodenal ulcer, and coronary artery disease. A thorough, structured evaluation of the patient’s symptoms is essential before any therapy, particularly any form of esophageal surgery. The presence and severity of both typical symptoms of heartburn, regurgitation, and dysphagia, and atypical symptoms of cough, hoarseness, chest pain, asthma, and aspiration should be discussed with the patient in detail. Many of these atypical symptoms may not be esophageal related and hence will not improve and may even worsen with antireflux surgery. Heartburn is generally defined as a substernal burningtype discomfort, beginning in the epigastrium and radiating upward. It is often aggravated by meals, spicy or fatty foods, chocolate, alcohol, and coffee and can be worse in the supine position. It is commonly, although not universally, relieved by antacid or antisecretory medications. Epidemiologic studies have shown that heartburn occurs monthly in as many as 40% 1031 American Gastroenterologic Association Gallup poll on nighttime gastroesophageal reflux disease symptoms • 50 million Americans have nighttime heartburn at least 1/wk • 80% of heartburn sufferers had nocturnal symptoms—65% both day & night • 63% report that it affects their ability to sleep and impacts their work the next day • 72% are on prescription medications • Nearly half (45%) report that current remedies do not relieve all symptoms to 50% of the Western population. The occurrence of heartburn at night and its effect on quality of life have recently been highlighted by a Gallup poll conducted by the American Gastroenterologic Society (Table 25-4). Regurgitation, the effortless return of acid or bitter gastric contents into the chest, pharynx, or mouth, is highly suggestive of foregut pathology. It is often particularly severe at night when supine or when bending over and can be secondary to either an incompetent or obstructed GEJ. With the latter, as in achalasia, the regurgitant is often bland, as if food was put into a blender. When questioned, most patients can distinguish the two. It is the regurgitation of gastric contents that may result in associated pulmonary symptoms, including cough, hoarseness, asthma, and recurrent pneumonia. Bronchospasm can be precipitated by esophageal acidification and cough by either acid stimulation or distention of the esophagus. Dysphagia, or difficulty swallowing, is a relatively nonspecific term but arguably the most specific symptom of foregut disease. It can be a sign of underlying malignancy and should be aggressively investigated until a diagnosis is established. Dysphagia refers to the sensation of difficulty in the passage of food from the mouth to the stomach and can be divided into oropharyngeal and esophageal etiologies. Oropharyngeal dysphagia is characterized by difficulty transferring food out of the mouth into the esophagus, nasal regurgitation, and/or aspiration. Esophageal dysphagia refers to the sensation of food sticking in the lower chest or epigastrium. This may or may not be accompanied by pain (odynophagia) that will be relieved by the passage of the bolus. Chest pain, although commonly and appropriately attributed to cardiac disease, is frequently secondary to esophageal pathology as well. Nearly 50% of patients with severe chest pain, normal cardiac function, and normal coronary arteriograms have positive 24-hour pH studies, implicating gastroesophageal reflux as the underlying etiology. Exercise-induced gastroesophageal reflux is well known to occur, and may result in exertional chest pain similar to angina. It can be quite difficult, if not impossible, to distinguish between the two etiologies, particularly on clinical grounds alone. Nevens and colleagues evaluated the ability of experienced cardiologists to differentiate pain of cardiac vs. esophageal origin. Of 248 patients initially seen by cardiologists, 185 were thought to have typical angina, and 63 were thought to have atypical chest pain. Forty-eight (26%) of those thought to have classic angina had normal coronary angiograms, and 16 of the 63 with atypical pain had abnormal angiogram. Thus, the cardiologists’ clinical impression was wrong 25% of the time. Finally, Pope and associates investigated the ultimate diagnosis in 10,689 patients presenting to an CHAPTER 25 ESOPHAGUS AND DIAPHRAGMATIC HERNIA Gastric Emptying Study. Gastric emptying studies are performed with radionuclide-labeled meals. Emptying of solids and liquids can be assessed simultaneously when both phases are marked with different tracers. After ingestion of a labeled standard meal, gamma camera images of the stomach are obtained at 5- to 15-minute intervals for 2 to 4 hours. After correction for decay, the counts in the gastric area are plotted as the percentage of total counts at the start of the imaging. The resulting emptying curve can be compared with data obtained in normal volunteers. In general, normal subjects will empty 59% of a meal within 90 minutes. Although delayed gastric emptying is often associated with gastroesophageal reflux, in general delayed emptying does not correlate with a poorer clinical outcome after antireflux surgery, and it should not be considered a contraindication to surgical treatment. Table 25-4 PART II SPECIFIC CONSIDERATIONS emergency department with acute chest pain. Approximately 17% were found to have acute ischemia, 6% had stable angina, 21% had other cardiac causes, and 55% had noncardiac causes. The investigators concluded that the majority of people presenting to the emergency department with chest pain do not have an underlying cardiac etiology for their symptoms. Chest pain precipitated by meals, occurring at night while supine, nonradiating, responsive to antacid medication, or accompanied by other symptoms suggesting esophageal disease such as dysphagia or regurgitation should trigger the thought of possible esophageal origin. Furthermore, the distinction between heartburn and chest pain is also difficult and largely dependent upon the individual patient. One person’s heartburn is another’s chest pain. The precise mechanisms accounting for the generation of symptoms secondary to esophageal pathology remain unclear. Considerable insight has been acquired, however. Investigations into the effect of luminal content, esophageal distention and muscular function, neural pathways, and brain localization have provided a basic understanding of the stimuli responsible for symptom generation. It is also clear that the visceroneural pathways of the foregut are complexly intertwined with that of the tracheobronchial tree and heart. This fact accounts for the common overlap of clinical presentations with diverse disease processes in upper GI, cardiac, and pulmonary systems. The Human Antireflux Mechanism and the Pathophysiology of Gastroesophageal Reflux Disease There is a high-pressure zone located at the esophagogastric junction in humans. Although this is typically referred to as the lower esophageal “sphincter,” there are no distinct anatomical landmarks that define its beginning and end. Architecturally speaking, there is a specialized thickening in this region that is made up of the collar sling musculature and the clasp fibers. The collar sling is located on the greater curvature side of the junction, and the clasp fibers are located on the lesser curvature side. These muscles remain in tonic opposition until the act of swallowing, whereupon receptive relaxation occurs allowing passage of a food bolus into the stomach. In addition, the LES will also open when the gastric fundus is distended with gas and liquid, thus resulting in an unfolding of the valve and enabling venting of gas (a belch). Whether physiologic or pathologic, the common denominator for most episodes of gastroesophageal reflux is the loss of the highpressure zone and thus a decrease in the resistance it imparts to the retrograde flow of gastric juice into the esophageal body. Table 25-5 Normal manometric values of the distal esophageal sphincter, n = 50 PARAMETER MEDIAN 2.5TH 97.5TH VALUE PERCENTILE PERCENTILE Pressure (mmHg) 13 5.8 27.7 Overall length (cm) 3.6 2.1 5.6 0.9 4.7 Abdominal length (cm) 2 A third characteristic of the LES that impacts its ability to prevent reflux is its position about the diaphragm. It is important that a portion of the total length of the LES be exposed to the effects of an intra-abdominal pressure. That is, during periods of elevated intra-abdominal pressure, the resistance of the barrier would be overcome if pressure were not applied equally to both the LES and stomach simultaneously. Thus, in the presence of a hiatal hernia, the sphincter resides entirely within the chest cavity and cannot respond to an increase in intra-abdominal pressure because the pinch valve mechanism is lost and gastroesophageal reflux is more liable to occur. Therefore, a permanently defective sphincter is defined by one or more of the following characteristics: an LES with a mean resting pressure of less than 6 mmHg, an overall sphincter length of <2 cm, and intra-abdominal sphincter length of <1 cm. Compared to normal subjects without GERD these values are below the 2.5 percentile for each parameter. The most common cause of a defective sphincter is an inadequate abdominal length. Once the sphincter is permanently defective, this condition is irreversible, and although esophageal mucosal injury may be healed with antisecretory medication, reflux will continue to occur. Additionally, the presence of a defective LES may be associated with reduced esophageal body function and thus decrease clearance times of refluxed material. In addition, the progressive loss of effective esophageal clearance may predispose the patient to severe mucosal injury, volume regurgitation, aspiration, and pulmonary injury. Reflux may occur in the face of a normal LES resting pressure. This condition is usually due to a functional problem of gastric emptying or excessive air swallowing. These conditions may lead to gastric distention, increased intra-gastric pressure, a resultant shortening or The Lower Esophageal Sphincter. As defined by esophageal manometry, there are three characteristics of the LES that work in unison to maintain its barrier function. These characteristics include the resting LES pressure, its overall length, and the intra-abdominal length that is exposed to the positive pressure environment of the abdomen (Table 25-5). The resistance to gastroesophageal reflux is a function of both the resting LES pressure and length over which this pressure is exerted. Thus, as the sphincter becomes shorter, a higher pressure will be required in order to prevent a given amount of reflux (Fig. 25-26). Much like the neck of a balloon as it is inflated, as the stomach fills and distends, sphincter length decreases. Therefore, if the overall length of the sphincter is permanently short from repeated distention of the fundus secondary to large volume meals, then with minimal episodes of gastric distention and pressure, there will be insufficient sphincter length for the barrier to remain competent, and reflux will occur. 24 LES pressure (mmHg) 1032 18 Competent 12 Incompetent 6 0 0 1 2 3 LES length (cm) 4 5 Figure 25-26. As the esophageal sphincter becomes shorter, increased pressure is necessary to maintain competence. LES = lower esophageal sphincter. Relationship Between Hiatal Hernia and Gastroesophageal Reflux Disease. As the collar sling musculature and clasp fibers become attenuated with repeated gastric distention, the esophagogastric junction begins to assume an “upside down funnel” appearance, with progressive opening of the acute angle of His. This in turn may result in attenuation and stretching of the phrenoesophageal ligament, with subsequent enlargement of the hiatal opening and axial herniation. There is a high degree of correlation between reflux threshold and the degree of hiatal herniation (Fig. 25-27). Summary. It is believed that GERD has its origins within the stomach. Distention of the fundus occurs because of overeating and delayed gastric emptying secondary to a high-fat diet. The resultant distention causes “unrolling” of the sphincter by the expanding fundus, and this subsequently exposes the squamous epithelium in the region of the distal LES to gastric juice. Repeated exposure results in inflammation and the development of columnar epithelium at the cardia. This is the initial step of the development of carditis and explains why in early disease esophagitis is mild and commonly limited to the very distal aspect of the esophagus. The patient attempts to compensate for this by increased swallowing, allowing the saliva to neutralize the refluxed gastric juice and thus, alleviate the discomfort induced by the reflux event. The increased swallowing results in aerophagia, bloating, and belching. This in turn creates a vicious cycle of increased gastric distention and thus further exposure and repetitive injury to the distal esophagus. The development of carditis explains the complaint of epigastric pain often experienced by patients with early reflux disease. Additionally, this process can lead to a fibrotic mucosal ring located at the squamocolumnar junction, which is termed a “Schatzki ring” and which may result in dysphagia. This inflammatory process may extend into muscularis propria and thus result in a progressive loss in the length and pressure of the LES. This explanation for the pathophysiology of GERD is supported by the observation that severe esophagitis is almost always associated with a defective LES. Complications Associated With Gastroesophageal Reflux Disease The complications of gastroesophageal reflux disease may result from the direct injurious effects of gastric fluid on the mucosa, larynx, or respiratory epithelium. Complications due to repetitive reflux are esophagitis, stricture, and BE; repetitive aspiration may lead to progressive pulmonary fibrosis. The severity of the complications is directly related to the prevalence of a structurally defective sphincter (Table 25-6). The observation that a structurally defective sphincter occurs in 42% of patients without complications (most of whom have one or two components failed) suggests that disease may be confined to the sphincter due to compensation by a vigorously contracting esophageal body. Eventually, all three components of the sphincter fail, allowing unrestricted reflux of gastric juice into the esophagus and overwhelming its normal clearance mechanisms. This leads to esophageal mucosal injury with progressive deterioration of esophageal contractility, as is commonly seen in patients with strictures and BE. The loss of esophageal clearance increases the potential for regurgitation into the pharynx with aspiration. Table 25-6 Complications of gastroesophageal reflux disease: 150 consecutive cases with proven gastroesophageal reflux disease (24-hour esophageal pH monitoring endoscopy, and motility) 40 36 Yield pressure (mmHg) 32 24 COMPLICATION NO. STRUCTURALLY NORMAL SPHINCTER (%) 20 None 59 58 42 16 Erosive esophagitis 47 23 77a 12 Stricture 19 11 89 8 Barrett’s esophagus 25 0 100 Total 150 28 4 0 No hernia < 3 cm hernia 3 cm hernia Figure 25-27. Yield pressure of the lower esophageal sphincter decreases as hiatal hernia size increases. STRUCTURALLY DEFECTIVE SPHINCTER (%) Grade more severe with defective cardia. Reproduced with permission from Moody FG, Carey LC, Jones RS, et al: Surgical Treatment of Digestive Disease. Chicago, IL: Year Book Medical; 1990. a 1033 CHAPTER 25 ESOPHAGUS AND DIAPHRAGMATIC HERNIA unfolding of the LES, and subsequent reflux. The mechanism by which gastric distention contributes to LES unfolding provides a mechanical explanation for “transient LES relaxation.” It is thought that with repeated gastric distention secondary to large meal volume or chronic air swallowing, there is repeated unfolding of the LES and subsequent attenuation of the collar sling musculature. It is at this point that the physiologic and normal mechanism of gastric venting is replaced with pathologic and severe postprandial reflux disease. In addition, patients with GERD will increase the frequency of swallowing in an effort to neutralize the refluxed acid with their saliva (pH 7.0). This phenomenon leads to increased air swallowing and further gastric distention, thus compounding the problem. Therefore, GERD may have its origins in the stomach secondary to gastric distention due to overeating/drinking, air swallowing, or consumption of carbonated liquids, and this may be further compounded by the ingestion of fatty meals, which result in delayed gastric emptying. 70 Prevalence 60 50 % 40 30 20 10 0 Gastric reflux (n = 22) Mixed reflux (n = 31) A 20 % Time SPECIFIC CONSIDERATIONS 15 10 5 0 pH <4 pH 4–7 pH >7 B Figure 25-29. A. Prevalence of reflux types in 53 patients with gastroesophageal reflux disease. B. Esophageal luminal pH during bilirubin exposure. (Reproduced with permission from Kauer WK, Peters JH, DeMeester TR, etal: Mixed reflux of gastric and duodenal juices is more harmful to the esophagus than gastric juice alone. The need for surgical therapy re-emphasized, Ann Surg. 1995 Oct;222(4):525-531.) Prevalence of patients with increased bilirubin 350 10 9 300 80 8 250 7 200 6 150 4 5 60 3 100 50 0 100 pH PART II The potential injurious components that reflux into the esophagus include gastric secretions such as acid and pepsin, as well as biliary and pancreatic secretions that regurgitate from the duodenum into the stomach. There is a considerable body of experimental evidence to indicate that maximal epithelial injury occurs during exposure to bile salts combined with acid and pepsin. These studies have shown that while acid alone does minimal damage to the esophageal mucosa, the combination of acid and pepsin is highly deleterious. Similarly, the reflux of duodenal juice alone does little damage to the mucosa, although the combination of duodenal juice and gastric acid is particularly noxious. Complications of gastroesophageal reflux such as esophagitis, stricture, and Barrett’s metaplasia occur in the presence of two predisposing factors: a mechanically defective LES and an increased esophageal exposure to fluid containing duodenal content that includes bile and pancreatic juice. The duodenal origin of esophageal contents in patients with an increased exposure to a pH >7 has previously been confirmed by esophageal aspiration studies (Fig. 25-28). Studies have clarified and expanded these observations by measuring esophageal bilirubin exposure over a 24-hour period as a marker for the presence of duodenal juice. Direct measurement of esophageal bilirubin exposure as a marker for duodenal juice has shown that 58% of patients with GERD have increased esophageal exposure to duodenal juice and that this exposure occurs most commonly when the esophageal pH is between 4 and 7 (Fig. 25-29). These earlier studies have been confirmed by other studies that measure volume reflux using impedance technology (Fig. 25-30). If reflux of gastric juice is allowed to persist and sustained or repetitive esophageal injury occurs, two sequelae can result. First, a luminal stricture can develop from submucosal and eventually intramural fibrosis. Second, the tubular esophagus may become replaced with columnar epithelium. The columnar epithelium is resistant to acid and is associated with the alleviation of the complaint of heartburn. This columnar epithelium often becomes intestinalized, identified histologically by the presence Bile acid conc. umol/l 1034 18:00 06:00 2 1 0 Time Figure 25-28. Sample bile acid concentration and esophageal pH plotted against time to obtain detailed profiles; in this case showing both significant bile acid (vertical bars) and acid (linear plot) reflux. (Reproduced with permission from Nehra D, Watt P, Pye JK, et al. Automated oesophageal reflux sampler: a new device used to monitor bile acid reflux in patients with gastroesophageal reflux disease, J Med Eng Technol. 1997 Jan-Feb;21(1):1-9.) 40 20 0 Normal subjects n = 25 No mucosal Erosive injury esophagitis n = 16 n = 10 Barrett’s esophagus n = 27 Figure 25-30. Prevalence of abnormal esophageal bilirubin exposure in healthy subjects and in patients with gastroesophageal reflux disease with varied degrees of mucosal injury. (*P <.03 vs. all other groups; **P <.03 vs. healthy subjects.) (Reproduced with permission from Kauer WK, Peters JH, DeMeester TR, et al: Mixed reflux of gastric and duodenal juices is more harmful to the esophagus than gastric juice alone. The need for surgical therapy re-emphasized, Ann Surg. 1995 Oct;222(4):525-531.) Metaplastic (Barrett’s Esophagus) and Neoplastic (Adenocarcinoma) Complications The condition whereby the tubular esophagus is lined with columnar epithelium rather than squamous epithelium was first by Norman Barrett in 1950. He incorrectly 3 described believed it to be congenital in origin. It is now realized that it is an acquired abnormality, occurs in 10% to 15% of patients with GERD, and represents the end stage of the natural history of this disease. It is also distinctly different from the congenital condition in which islands of gastric fundic epithelium are found in the upper half of the esophagus. The definition of BE has evolved considerably over the past decade. Traditionally, BE was identified by the presence of columnar mucosa extending at least 3 cm into the esophagus. It is now recognized that the specialized, intestinal-type epithelium, or intestinal metaplasia (IM) found in the Barrett’s mucosa, is the only tissue predisposed to malignant degeneration. Consequently, the diagnosis of BE is presently made given any length of endoscopically identifiable columnar mucosa that proves, on biopsy, to show IM. Although long segments of columnar mucosa without IM do occur, they are uncommon and might be congenital in origin. The hallmark of IM is the presence of intestinal goblet cells. There is a high prevalence of biopsy-demonstrated IM at the cardia, on the gastric side of the squamocolumnar junction, in the absence of endoscopic evidence of a CLE. Evidence is accumulating that these patches of what appears to be Barrett’s in the cardia have a similar malignant potential as in the longer segments, and are precursors for carcinoma of the cardia. The long-term relief of symptoms remains the primary reason for performing antireflux surgery in patients with BE. Healing of esophageal mucosal injury and the prevention of disease progression are important secondary goals. In this regard, patients with BE are no different than the broader population of patients with gastroesophageal reflux. They should be considered for antireflux surgery when patient data suggest severe disease or predict the need for long-term medical management. Most patients with BE are symptomatic. Although it has been argued that some patients with BE may not have symptoms, careful history taking will reveal the presence of symptoms in most, if not all, patients. Patients with BE have a spectrum of disease ranging from visually identifiable but short segments, to long segments of classic BE. In general, however, they represent a relatively severe stage of gastroesophageal reflux, usually with markedly increased esophageal acid exposure, deficient LES characteristics, poor esophageal body function, and a high prevalence of duodenogastroesophageal reflux. Gastric hypersecretion occurs in 44% of patients. Most will require long-term PPI therapy for relief of symptoms and control of coexistent esophageal mucosal injury. Given such profound deficits in esophageal physiology, antireflux surgery is an excellent means of long-term control of reflux symptoms for most patients with BE. The typical complications in BE include ulceration in the columnar-lined segment, stricture formation, and a dysplasiacancer sequence. Barrett’s ulceration is unlike the erosive ulceration of reflux esophagitis in that it more closely resembles peptic ulceration in the stomach or duodenum, and has the same propensity to bleed, penetrate, or perforate. Fortunately, this complication occurs very rarely. The strictures found in BE occur at the squamocolumnar junction, and they are typically higher than peptic strictures in the absence of BE. Ulceration and stricture in association with BE were commonly reported before 1975, but with the advent of potent acid suppression medication, they have become less common. In contrast, the complication of adenocarcinoma developing in Barrett’s mucosa has become more common. Adenocarcinoma developing in Barrett’s mucosa was considered a rare tumor before 1975. Today, it occurs at approximately 0.2% to 0.5% per year of followup, which represents a risk 40 times that of the general population. Most, if not all, cases of adenocarcinoma of the esophagus arise in Barrett’s epithelium (Fig. 25-31). About one-third of all patients with BE present with malignancy. The long-term risk of progression to dysplasia and adenocarcinoma, although not the driving force behind the decision to perform antireflux surgery, is a significant concern for both patient and physician. Although to date, there have been no prospective randomized studies documenting that antireflux surgery has an effect on the risk of progression to dysplasia and carcinoma, complete control of reflux of gastric juice into the esophagus is clearly a desirable goal. Respiratory Complications A significant proportion of patients with GERD will have associated respiratory symptoms. These patients may have laryngopharyngeal reflux-type symptoms, adult-onset asthma, or even idiopathic pulmonary fibrosis. These symptoms and organ injury may occur in isolation or in conjunction with typical reflux symptoms such as heartburn and regurgitation. Several studies have demonstrated that up to 50% of patients with asthma have either endoscopically evident esophagitis or abnormal distal esophageal acid exposure. These findings support a causal relationship between GERD and aerodigestive symptoms and complications in a proportion of patients. 1035 CHAPTER 25 ESOPHAGUS AND DIAPHRAGMATIC HERNIA of goblet cells. This specialized IM is currently required for the diagnosis of BE. Endoscopically, BE can be quiescent or associated with complications of esophagitis, stricture, Barrett’s ulceration, and dysplasia. The complications associated with BE may be due to the continuous irritation from refluxed duodenogastric juice. This continued injury is pH dependent and may be modified by medical therapy. The incidence of metaplastic Barrett’s epithelium becoming dysplastic and progressing to adenocarcinoma is approximately 0.2% to 0.5% per year. An esophageal stricture can be associated with severe esophagitis or BE. In the latter situation, it occurs at the site of maximal inflammatory injury (i.e., the columnar-squamous epithelial interface). Patients who have a stricture in the absence of Barrett’s esophagus should have the presence of gastroesophageal reflux documented before the presence of the stricture is ascribed to reflux esophagitis. In patients with normal acid exposure and no endoscopic or CT evidence of cancer, the stricture may be a result of a drug-induced chemical injury, the latter resulting from the lodgment of a capsule or tablet in the distal esophagus. In such patients, dilation usually corrects the problem of dysphagia. It is also possible for drug-induced injuries to occur in patients who have underlying esophagitis and a distal esophageal stricture secondary to gastroesophageal reflux. In this situation, a long, string-like stricture progressively develops as a result of repetitive caustic injury from capsule or tablet lodgment on top of an initial reflux stricture. These strictures are often resistant to dilation. The incidence of this problem has lessened since the introduction of proton pump inhibitor medication. 1036 PART II SPECIFIC CONSIDERATIONS A B Figure 25-31. Photomicrographs. A. Barrett’s epithelium with severe dysplasia. (×200.) Note nuclear irregularity, stratification, and loss of polarity. B. Barrett’s epithelium with intramucosal carcinoma. (×66.) Note malignant cells in the mucosa (upper arrow), but not invading the muscularis mucosae (bottom arrow). (Reproduced with permission from Zuidema GD, Orringer MB: Shackelford’s Surgery of the Alimentary Tract, 3rd ed. Vol 1. Philadelphia, PA: Elsevier/Saunders; 1991.) Etiology of Reflux-Induced Respiratory Symptoms. There are two mechanisms that have been proposed as the cause of reflux-induced respiratory symptoms. The reflux theory suggests that these symptoms are the direct result of laryngopharyngeal exposure and aspiration of gastric contents. The reflex theory suggests that the vagal-mediated afferent fibers result in bronchoconstriction during episodes of distal esophageal acidification. The evidence supporting a mechanism of direct exposure to the aerodigestive system is based in clinical studies that have documented a strong correlation between idiopathic pulmonary fibrosis and hiatal hernia. In addition, the presence of GERD was demonstrated to be highly associated with several pulmonary diseases in a recent Department of Veteran Affairs multivariate analysis. Next, with ambulatory pH testing, acid exposure within the proximal esophagus is more frequently identified in patients with gastroesophageal reflux and respiratory symptoms than in patients who have gastroesophageal reflux symptoms alone. These findings are supported by scintigraphic studies, which have demonstrated aspiration of ingested radioisotope in patients with both gastroesophageal reflux and pulmonary symptoms. In animal studies, tracheal instillation of acid has been demonstrated to profoundly increase airway resistance. Finally, in patients who have undergone multichannel intraluminal impedance testing with a catheter configured to detect laryngopharyngeal reflux, a correlation between proximal fluid movement and laryngopharyngeal symptoms, such as cough, can be demonstrated. The reflex mechanism is supported by the bronchoconstriction that occurs with the infusion of acid into the distal esophagus. There is a shared embryologic origin of the tracheoesophageal tract and vagus nerve, and this reflex is thought to be an afferent fiber–mediated reflex that protects the aerodigestive system from the aspiration of refluxate. In patients with respiratory symptoms and documented gastroesophageal reflux without proximal esophageal acid exposure, pulmonary symptoms will often times significantly improve or completely resolve after undergoing laparoscopic fundoplication. It is likely that both of the proposed mechanisms work simultaneously to cause these symptoms in the face of GERD. The most difficult clinical challenge in formulating a treatment plan for reflux-associated respiratory symptoms resides in establishing the diagnosis. Although the diagnosis may be straightforward in patients with predominately typical reflux symptoms and secondary respiratory complaints, a substantial number of patients will have respiratory symptoms that dominate the clinical scenario. Typical gastroesophageal reflux Treatment. Once the diagnosis is established, treatment may be initiated with either PPI therapy or antireflux surgery. A trial of high-dose PPI therapy may help establish that reflux is partly or completely responsible for the respiratory symptoms. It is important to note that the persistence of symptoms in the face of aggressive PPI treatment does not necessarily rule out reflux as a possible cofactor or sole etiology. Although there is probably some element of a placebo effect, relief of respiratory symptoms can be anticipated in up to 50% of patients with reflux-induced asthma treated with antisecretory medications. However, when examined objectively, <15% of patients can be expected to have improvement in their pulmonary function with medical therapy. In properly selected patients, antireflux surgery improves respiratory symptoms in nearly 90% of children and 70% of adults with asthma and reflux disease. Improvements in pulmonary function can be demonstrated in around 30% of patients. Uncontrolled studies of the two forms of therapy (PPI and surgery) and the evidence from the two randomized controlled trials of medical vs. surgical therapy indicate that surgical valve reconstruction is the most effective therapy for reflux-induced asthma. The superiority of the surgery over PPI is most noticeable in the supine position, which corresponds with the nadir of PPI blood levels and resultant acid breakthrough and is the time in the circadian cycle when asthma symptoms are at their worst. In asthmatic patients with an esophageal motility disorder, performing an antireflux operation will not prevent the regurgitation and possible aspiration of swallowed liquid or food “upstream” to the valve reconstruction. It is critical that esophageal body function be considered prior to surgical intervention in this patient population. Medical Therapy for Gastroesophageal Reflux Disease. With the widespread availability of over-the-counter antisecretory medications, most patients with mild or moderate symptoms will carry self-medication. When initially identified with mild symptoms of uncomplicated GERD, patients can be placed on 12 weeks of simple antacids before diagnostic testing is initiated. This approach may successfully and completely resolve the symptoms. Patients should be counseled to elevate the head of the bed; avoid tight-fitting clothing; eat small, frequent meals; avoid eating the nighttime meal immediately prior to bedtime; and avoid alcohol, coffee, chocolate, and peppermint, which are known to reduce resting LES pressure and may aggravate symptoms. Used in combination with simple antacids, alginic acid may augment the relief of symptoms by creating a physical barrier to reflux, as well as by acid reduction. Alginic acid reacts with sodium bicarbonate in the presence of saliva to form a highly viscous solution that floats like a raft on the surface of the gastric contents. When reflux occurs, this protective layer is refluxed into the esophagus, and acts as a protective barrier against the noxious gastric contents. Medications to promote gastric emptying, such as metoclopramide or domperidone, are beneficial in early disease but of little value in more severe disease. In patients with persistent symptoms, the mainstay of medical therapy is acid suppression. High-dosage regimens of hydrogen potassium PPIs, such as omeprazole (up to 40 mg/d), can reduce gastric acidity by as much as 80% to 90%. This usually heals mild esophagitis. In severe esophagitis, healing may occur in only one-half of the patients. In patients who reflux a combination of gastric and duodenal juice, acid-suppression therapy may give relief of symptoms, while still allowing mixed reflux to occur. This can allow persistent mucosal damage in an asymptomatic patient. Unfortunately, within 6 months of discontinuation of any form of medical therapy for GERD, 80% of patients have a recurrence of symptoms, and 40% of individuals with daily GERD eventually develop symptoms that “breakthrough” adequately dosed PPIs. Once initiated, most patients with GERD will require lifelong treatment with PPIs, both to relieve symptoms and to control any coexistent esophagitis or stricture. Although control of symptoms has historically served as the endpoint of therapy, the wisdom of this approach has recently been questioned, particularly in patients with BE. Evidence suggesting that reflux control may prevent the development of adenocarcinoma and lead to regression of dysplastic and nondysplastic Barrett’s segments has led many to consider control of reflux, and not symptom control, a better therapeutic endpoint. However, this hypothesis remains controversial. It should be noted that complete control of reflux using PPIs can be difficult, as has been highlighted by studies of acid breakthrough while on PPI therapy and of persistent reflux following antireflux surgery. Castell, Triadafilopoulos, and others have shown that 40% to 80% of patients with BE continue to have abnormal esophageal acid exposure despite up to 20 mg twice daily of PPIs. Ablation trials have shown that mean doses of 56 mg of omeprazole were necessary to normalize 24-hour esophageal pH studies. It is likely that antireflux surgery results in more reproducible and reliable elimination of reflux of both acid and duodenal contents, although long-term outcome studies suggest that as many as 25% of postfundoplication patients will have persistent pathologic esophageal acid exposure confirmed by positive 24-hour pH studies. Suggested Therapeutic Approach. Traditionally a stepwise approach is used for the treatment of GERD. First-line therapy entails antisecretory medication, usually PPIs, in most patients. Failure of medication to adequately control GERD symptoms suggests either that the patient may have relatively severe disease or a non-GERD cause for his or her symptoms. Endoscopic examination at this stage of the patient’s evaluation is recommended and will provide the opportunity to assess the degree of mucosal injury and presence of BE. Treatment options for these patients entails either long term PPI use vs. antireflux surgery. Laparoscopic antireflux surgery in these patients achieves longterm control of symptoms in 85% to 90%. The measurement 1037 CHAPTER 25 ESOPHAGUS AND DIAPHRAGMATIC HERNIA symptoms, such as heartburn and regurgitation, may often be completely absent only to be uncovered with objective esophageal physiology testing. Traditionally, the diagnosis of refluxinduced respiratory injury is established using ambulatory dual probe pH monitoring, with one probe positioned within the distal esophagus and the other at a proximal location. Proximal probe positioning has included multiple locations such as the trachea, pharynx, and proximal esophagus. Although ambulatory esophageal pH monitoring allows a direct correlation between esophageal acidification and respiratory symptoms, sensitivity of this testing modality is poor, and the temporal relationship between laryngeal or pulmonary symptoms and reflux events is complex. In addition, as the refluxed gastric fluid travels proximally, it may be neutralized by saliva and therefore go undetected with pH monitoring. Impedance testing may also be used to detect the movement of fluid throughout the entire esophageal column regardless of pH content. 1038 of esophageal acid exposure via 24-hour pH should be undertaken when patients are considered for surgery. The status of the LES and esophageal body function with esophageal manometry should also be performed at this stage. These studies will serve to establish the diagnosis and assess esophageal body dysfunction. Surgical Therapy for Gastroesophageal Reflux Disease Selection of Patients for Surgery. Studies of the natural PART II SPECIFIC CONSIDERATIONS history of GERD indicate that most patients have a relatively benign form of the disease that is responsive to lifestyle changes and dietary and medical therapy and do not need surgical treatment. Approximately 25% to 50% of the patients with GERD have persistent or progressive disease, and it is this patient population that is best suited to surgical therapy. In the past, the presence of esophagitis and a structurally defective LES were the primary indications for surgical treatment, and many internists and surgeons were reluctant to recommend operative procedures in their absence. However, one should not be deterred from considering antireflux surgery in a symptomatic patient with or without esophagitis or a defective sphincter, provided the disease process has been objectively documented by 24-hour pH monitoring. This is particularly true in patients who have become dependent upon therapy with PPIs, or require increasing doses to control their symptoms. It is important to note that a good response to medical therapy in this group of patients predicts an excellent outcome following antireflux surgery. In general, the key indications for antireflux surgery are (a) objectively proven gastroesophageal reflux disease, and (b) typical symptoms of gastroesophageal reflux disease (heartburn and/or regurgitation) despite adequate medical management, or (c) a younger patient unwilling to take lifelong medication. In addition, a structurally defective LES can also predict which patients are more likely to fail with medical therapy. Patients with normal sphincter pressures tend to remain well controlled with medical therapy, whereas patients with a structurally defective LES may not respond as well to medical therapy, and often develop recurrent symptoms within 1 to 2 years of beginning therapy. Such patients should be considered for an antireflux operation, regardless of the presence or absence of endoscopic esophagitis. Young patients with documented reflux disease with or without a defective LES are also excellent candidates for antireflux surgery. They usually will require long-term medical therapy for control of their symptoms, and some will go on to develop complications of the disease. An analysis of the cost of therapy based on data from the Veterans Administration Cooperative trial indicates that surgery has a cost advantage over medical therapy in patients <49 years of age. Severe endoscopic esophagitis in a symptomatic patient with a structurally defective LES is also an indication for early surgical therapy. These patients are prone to breakthrough of their symptoms while receiving medical therapy. Symptoms and mucosal injury can be controlled in such patients, but careful monitoring is required, and increasing dosages of PPIs are necessary. In everyday clinical practice, however, such treatment can be both difficult and impractical, and, in such cases, antireflux surgery can be considered early, especially if PPI therapy is problematic. The development of a stricture in a patient represents a failure of medical therapy, and it is also an indication for a surgical antireflux procedure. In addition, strictures are often associated with a structurally defective sphincter and loss of esophageal contractility. Before proceeding with surgical treatment, malignancy and a drug-related etiology of the stricture should be excluded, and the stricture should be progressively dilated up to a 50 to 60F bougie. When the stricture is fully dilated, the relief of dysphagia is evaluated, and esophageal manometry is performed to determine the adequacy of peristalsis in the distal esophagus. If dysphagia is relieved and the amplitude of esophageal contractions is adequate, an antireflux procedure should be performed; if there is a global loss of esophageal contractility, caution should be exercised in performing an antireflux procedure with a complete fundoplication, and a partial fundoplication should be considered. Barrett’s CLE is commonly associated with a severe structural defect of the LES and often poor contractility of the esophageal body. Patients with BE are at risk of the development of an adenocarcinoma. Whilst surgeons would like to think that an antireflux procedure can reduce the risk of progression to cancer, the evidence supporting this is relatively weak, and for now Barrett’s esophagus should be considered to be evidence that the patient has gastroesophageal reflux, and progression to antireflux surgery is indicated for the treatment of reflux symptoms, not cancer progression. If, however, high grade dysplasia or intramucosal carcinoma is found on mucosal biopsy specimens, treatment should then be directed at the BE and the lesion, using either evaluation endoscopic ablation, endoscopic resection, or esophageal resection. The majority of patients requiring treatment for reflux have a relatively mild form of disease and will respond to antisecretory medications. Patients with more severe forms of disease, particularly those who develop persistent or progressive disease, should be considered for definitive therapy. Laparoscopic fundoplication will provide a long-term cure in the majority of these patients, with minimal discomfort and an early return to normal activity. Preoperative Evaluation. Before proceeding with an antireflux operation, several factors should be evaluated. The clinical symptoms should be consistent with the diagnosis of gastroesophageal reflux. Patients presenting with the typical symptoms of heartburn and/or regurgitation which have responded, at least partly, to PPI therapy, will generally do well following surgery, whereas patients with atypical symptoms have a less predictable response. Reflux should also be objectively confirmed by either the presence of ulcerative esophagitis or an abnormal 24-hour pH study. The propulsive force of the body of the esophagus should be evaluated by esophageal manometry to determine if it has sufficient power to propel a bolus of food through a newly reconstructed valve. Patients with normal peristaltic contractions can be considered for a 360° Nissen fundoplication or a partial fundoplication, depending on patient and surgeon preferences. When peristalsis is absent, a partial fundoplication is probably the procedure of choice, but only if achalasia has been ruled out. Hiatal anatomy should also be assessed. In patients with smaller hiatal hernias, endoscopy evaluation usually provides sufficient information. However, when patients present with a very large hiatus hernia or for revision surgery after previous antireflux surgery, contrast radiology provides better anatomical information. The concept of anatomic shortening of the esophagus is controversial, with divergent opinions held about how Principles of Surgical Therapy. The primary goal of antireflux surgery is to safely create a new antireflux valve at the gastroesophageal junction, while preserving the patient’s ability to swallow normally and to belch to relieve gaseous distention. Regardless of the choice of the procedure, this goal can be achieved if attention is paid to some basic principles when reconstructing the antireflux mechanism. First, the operation should create a flap valve which prevents regurgitation of gastric contents into the esophagus. This will result in an increase in the pressure of the distal esophageal sphincter region. Following a Nissen fundoplication the expected increase is to a level twice the resting gastric pressure (i.e., 12 mmHg for a gastric pressure of 6 mmHg). The extent of the pressure rise is often less following a partial fundoplication, although with all types of fundoplication the length of the reconstructed valve should be at least 3 cm. This not only augments sphincter characteristics in patients in whom they are reduced before surgery but also prevents unfolding of a normal sphincter in response to gastric distention (Fig. 25-32). Preoperative and postoperative esophageal manometry measurements have shown that the resting sphincter pressure and the overall sphincter length can be surgically augmented over preoperative values, and that the change in the former is a function of the degree of gastric wrap around the esophagus (Fig. 25-33). However, the aim of any fundoplication is to create a loose wrap and to maintain the position of the gastric fundus close to the distal intra-abdominal esophagus, in a flap valve arrangement. The efficacy of this relies on the close relationship between the fundus and the esophagus, not the “tightness” of the wrap. Second, the operation should place an adequate length of the distal esophageal sphincter in the positive-pressure Distention Figure 25-32. A graphic illustration of the shortening of the lower esophageal sphincter that occurs as the sphincter is “taken up” by the cardia as the stomach distends. ∆ P mmHg 20 Hill Belsey Nissen N=15 N=15 N=15 1039 15 10 5 Y = 4.63 + .023 (x) P < .01 0 240 Degree of wrap 360 Figure 25-33. The relationship between the augmentation of sphincter pressure over preoperative pressure (∆P) and the degree of gastric fundic wrap in three different antireflux procedures. (Reproduced with permission from O’Sullivan GC, DeMeester TR, Joelsson BE, et al: Interaction of lower esophageal sphincter pressure and length of sphincter in the abdomen as determinants of gastroesophageal competence, Am J Surg. 1982 Jan;143(1):40-47.) environment of the abdomen by a method that ensures its response to changes in intra-abdominal pressure. The permanent restoration of 2 or more cm of abdominal esophagus ensures the preservation of the relationship between the fundus and the esophagus. All of the popular antireflux procedures increase the length of the sphincter exposed to abdominal pressure by an average of at least 1 cm. Third, the operation should allow the reconstructed cardia to relax on deglutition. In normal swallowing, a vagally mediated relaxation of the distal esophageal sphincter and the gastric fundus occurs. The relaxation lasts for approximately 10 seconds and is followed by a rapid recovery to the former tonicity. To ensure relaxation of the sphincter, three factors are important: (a) Only the fundus of the stomach should be used to buttress the sphincter, because it is known to relax in concert with the sphincter; (b) the gastric wrap should be properly placed around the sphincter and not incorporate a portion of the stomach or be placed around the stomach itself, because the body of the stomach does not relax with swallowing; and (c) damage to the vagal nerves during dissection of the thoracic esophagus should be avoided because it may result in failure of the sphincter to relax. Fourth, the fundoplication should not increase the resistance of the relaxed sphincter to a level that exceeds the peristaltic power of the body of the esophagus. The resistance of the relaxed sphincter depends on the degree, length, and diameter of the gastric fundic wrap, and on the variation in intra-abdominal pressure. A 360° gastric wrap should be no longer than 2 cm and constructed over a large (50 to 60F) bougie. This will ensure that the relaxed sphincter will have an adequate diameter with minimal resistance. A bougie is not necessary when constructing a partial wrap. Fifth, the operation should ensure that the fundoplication can be placed in the abdomen without undue tension and maintained there by approximating the crura of the diaphragm above the repair. Leaving the fundoplication in the thorax converts a sliding hernia into a PEH, with all the complications associated with that condition. Maintaining the repair in the abdomen CHAPTER 25 ESOPHAGUS AND DIAPHRAGMATIC HERNIA common this problem is. Believers claim that anatomic shortening of the esophagus compromises the ability of the surgeon to perform an adequate repair without tension and that this can lead to an increased incidence of breakdown or thoracic displacement of the repair. Some of those who hold this view claim that esophageal shortening is present when a barium swallow X-ray identifies a sliding hiatal hernia that will not reduce in the upright position or that measures more than 5 cm in length at endoscopy. When such identification is made, these surgeons usually add a gastroplasty to the antireflux procedure. Others claim that esophageal shortening is overdiagnosed and rarely seen, and that the morbidity of adding a gastroplasty outweighs any benefits. These surgeons would recommend a standard antireflux procedure in all patients undergoing primary surgery. 1040 under tension predisposes to an increased incidence of recurrence. How common this problem is encountered is disputed, with some surgeons advocating lengthening the esophagus by gastroplasty and constructing a partial fundoplication, and others claiming that this issue is now rarely encountered. PART II SPECIFIC CONSIDERATIONS Procedure Selection. A laparoscopic approach is now used routinely in all patients undergoing primary antireflux surgery. Some surgeons advocate the use of a single antireflux procedure for all patients, whereas others advocate a tailored approach. Advocates of the laparoscopic Nissen fundoplication as the procedure of choice for a primary antireflux repair would generally apply this procedure in all patients with normal or near normal esophageal motility, and they would reserve a partial fundoplication for use in individuals with poor esophageal body motility. Others, based on the good longer-term outcomes now reported following partial fundoplication procedures, advocate the routine application of a partial fundoplication procedure, thereby avoiding any concerns about constructing a fundoplication in individuals with poor esophageal motility. Experience and randomized studies have shown that both the Nissen fundoplication and various partial fundoplication procedures are all effective and durable antireflux repairs that generate an excellent outcome in approximately 90% of patients at longer-term follow-up. L R Primary Antireflux Repairs Nissen Fundoplication. The most common antireflux procedure is the Nissen fundoplication. In the past, this procedure has been performed through an open abdominal or a chest incision, but with the development of laparoscopic approaches primary antireflux surgery is now routinely undertaken using the laparoscope. Rudolph Nissen described this procedure as a 360° fundoplication around the lower esophagus for a distance of 4 to 5 cm, without division of the short gastric blood vessels. Although this provided good control of reflux, it was associated with a number of side effects that have encouraged modifications of the procedure as originally described. These include using only the gastric fundus to envelop the esophagus in a fashion analogous to a Witzel jejunostomy, sizing the fundoplication with a large (50 to 60F) bougie, limiting the length of the fundoplication to 1 to 2 cm, and dividing the short gastric vessels. The essential elements necessary for the performance of a transabdominal fundoplication are common to both the laparoscopic and open procedures and include the following: 1. Hiatal dissection and preservation of both vagi along their entire length 2. Circumferential esophageal mobilization 3. Hiatal closure, usually posterior to the esophagus 4. Creation of a short and floppy fundoplication over an esophageal dilator In addition, many surgeons also routinely divide the short gastric blood vessels, although this step is not universally applied, and the results of several randomized trials have failed to show that this step yields any benefit. The laparoscopic approach to fundoplication has now replaced the open abdominal Nissen fundoplication as the procedure of choice. Five ports are usually used (Fig. 25-34), and dissection is begun by incising the gastrohepatic omentum above and below the hepatic branch of the anterior vagus nerve, which is usually preserved. The circumference of the diaphragmatic Figure 25-34. Patient positioning and trocar placement for laparoscopic antireflux surgery. The patient is placed with the head elevated approximately 30° in the modified lithotomy position. The surgeon stands between the patient’s legs, and the procedure is completed using five abdominal access ports. hiatus is dissected and the esophagus is mobilized by careful dissection of the anterior and posterior soft tissues within the hiatus. The esophagus is held anterior and to the left and the hiatal pillars are approximated with interrupted nonabsorbable sutures, starting posteriorly and working anteriorly. A tension-free fundoplication should be constructed. This can usually be achieved either with or without division of the short gastric blood vessels, according to surgeon preference. If the vessels are divided, the upper one-third of the greater curvature is mobilized by sequentially dissecting and dividing these vessels, commencing distally and working proximally. Following complete fundal mobilization, the posterior wall of the fundus is brought behind the esophagus to the right side, and the anterior wall of the fundus is brought anterior to the esophagus. The fundic lips are manipulated to allow the fundus to envelop the esophagus without twisting. A 50 to 60F bougie is passed to properly size the fundoplication, and it is sutured using nonabsorbable sutures. Some surgeons use a single U-stitch of 2-0 polypropylene buttressed with felt pledgets (Fig. 25-35), and others use 2-4 interrupted sutures. Posterior Partial Fundoplication. Partial fundoplications were obstruction of a complete fundoplication. The Toupet posterior partial fundoplication consists of a 270° gastric fundoplication around the distal 4 cm of esophagus (Fig. 25-36). It is usually stabilized by anchoring the wrap posteriorly to the hiatal rim. 1041 Anterior Partial Fundoplication. An alternative approach to partial fundoplication is to construct an anterior partial fundoplication. Following posterior hiatal repair, the anterior fundus is rolled over the front of the esophagus and sutured to the hiatal rim and the esophageal wall. Division of the short gastric vessels Figure 25-35. A. Laparoscopic Nissen fundoplication is performed with a five-trocar technique. B. The liver retractor is affixed to a mechanical arm to hold it in place throughout the operation. C. After division of the gastrohepatic omentum above the hepatic branch of the vagus (pars flaccida), the surgeon places a blunt atraumatic grasper beneath the phrenoesophageal ligament. D. After completion of the crural closure, an atraumatic grasper is placed right to left behind the gastroesophageal junction. The grasper is withdrawn, pulling the posterior aspect of the gastric fundus behind the esophagus. E. Once the suture positions are chosen, the first stitch (2-0 silk, 20 cm long) is introduced through the 10-mm trocar, and the needle is passed first through the left limb of the fundus, then the esophagus (2.5 cm above the gastroesophageal junction), then through the right limb of the fundus. F. Final position of the fundoplication. CHAPTER 25 ESOPHAGUS AND DIAPHRAGMATIC HERNIA developed as an alternative to the Nissen procedure in an attempt to minimize the risk of postfundoplication side effects, such as dysphagia, inability to belch, and flatulence. The commonest approach has been a posterior partial or Toupet fundoplication. Some surgeons use this type of procedure for all patients presenting for antireflux surgery, whereas others apply a tailored approach in which a partial fundoplication is constructed in patients with impaired esophageal motility, in which the propulsive force of the esophagus is thought to be insufficient to overcome the outflow 1042 PART II SPECIFIC CONSIDERATIONS Figure 25-35. (Continued ) is never needed when constructing this type of fundoplication. Various degrees of anterior partial fundoplication have been described—90°, 120°, 180°. The anterior 180° partial fundoplication (Fig. 25-37) provides a more robust fundoplication and achieves an excellent longer-term outcome in approximately 90% of patients at follow-up of at least 10 years. With this procedure, the fundus and esophagus are sutured to the right side of the hiatal rim to create a flap valve at the gastroesophageal junction and to stabilize a 3 to 4 cm length of intra-abdominal esophagus. Collis Gastroplasty. When a shortened esophagus is encountered, many surgeons choose to add an esophageal lengthening procedure before fundoplication, to reduce the tension on the gastroesophageal junction, believing this will minimize the risk of failure due to postoperative hiatus hernia. The commonest approach to this is the Collis gastroplasty. This entails using a stapler to divide the cardia and upper stomach, parallel to the lesser curvature of Figure 25-36. Completed laparoscopic posterior partial (Toupet) fundoplication. The fundoplication does not cover the anterior surface of the esophagus, and it is stabilized by suturing the fundus to the side of the esophagus, and posteriorly to the right hiatal pillar. the stomach, thereby creating a gastric tube in continuity with the esophagus, and effectively lengthening the esophagus by several centimeters. Laparoscopic techniques for Collis gastroplasty have been described (Fig. 25-38). Following gastroplasty a fundoplication is constructed, with the highest suture is placed on the native esophagus when constructing a Nissen fundoplication. Not all surgeons choose to undertake a Collis procedure, however, as there is controversy about the actual incidence of the shortened esophagus and widely divergent views are held about how often this problem is encountered. In addition, some surgeons have questioned the wisdom of creating an amotile tube of gastric wall, which can secrete acid, and then placing a Nissen fundoplication below this. Outcome After Fundoplication. Studies of long-term outcome following both open and laparoscopic fundoplication document the ability of laparoscopic fundoplication to relieve typical reflux symptoms (heartburn, regurgitation, and dysphagia) in more than Figure 25-37. Completed laparoscopic anterior 180° partial fundoplication. The fundoplication fully covers the anterior surface of the esophagus, and it is stabilized by suturing the fundus to the right side of the esophagus, and to the right hiatal pillar. Unlike the Nissen procedure, the fundus is not pulled behind the esophagus. 1043 CHAPTER 25 ESOPHAGUS AND DIAPHRAGMATIC HERNIA Figure 25-38. A. After removal of the fat pad and release of tension on the Penrose drain, the gastroesophageal junction (GES) retracts to the level of the hiatus. The interior end of the staple line is marked 2/5 cm below the angle of His. B. The first horizontal firing of the stapler occurs by maximally articulating the stapler to the left, aiming toward the previously marked spot adjacent to the dilator. C. The vertical staple line is created by a single firing of the GIA placed parallel and flush against the 48F dilator. D. The highest Nissen fundoplication suture is placed on the native esophagus, and the second suture tucks in the apex of the staple line. 90% of patients at follow-up intervals averaging 2 to 3 years and 80% to 90% of patients 5 years or more following surgery. This includes evidence-based reviews of antireflux surgery, prospective randomized trials comparing antireflux surgery to PPI therapy and open to laparoscopic fundoplication and analysis of U.S. national trends in use and outcomes. Postoperative pH studies indicate that more than 90% of patients will normalize their pH tracings. The results of laparoscopic fundoplication compare favorably with those of the “modern” era of open fundoplication. They also indicate the less predictable outcome of atypical reflux symptoms (cough, asthma, laryngitis) after surgery, being relieved in only two-thirds of patients. The goal of surgical treatment for GERD is to relieve the symptoms of reflux by reestablishing the gastroesophageal barrier. The challenge is to accomplish this without inducing dysphagia or other untoward side effects. Dysphagia, existing before surgery, usually improves following laparoscopic fundoplication. Temporary dysphagia is common after surgery and generally resolves within 3 months, but it can take up to 12 months in some individuals, and dysphagia sufficient to require ongoing dietary modification persists in up to 5% of individuals following Nissen fundoplication. Other side effects common to antireflux surgery include the inability to belch and vomit and increased flatulence. Most patients cannot vomit through an intact wrap, though this is rarely clinically relevant. Most patients are unable to belch gas from the stomach in the first 3 to 6 months after fundoplication, but 80% to 90% regain the ability to belch normally beyond the first 12 months of follow-up. Hyperflatulence is a common and noticeable problem, likely related to increased air swallowing that is present in most patients with reflux disease, aggravated by the inability to belch in some patients. 1044 Randomized Controlled Trials Addressing Surgical Technique Division of the Short Gastric Blood Vessels Originally, PART II SPECIFIC CONSIDERATIONS Nissen’s description of a total fundoplication entailed a 360° fundoplication during which the short gastric blood vessels were left intact. However, with reports of troublesome postoperative dysphagia, division of these vessels—to achieve full fundal mobilization and thereby ensure a loose fundoplication—was promoted and has entered common practice. The evidence supporting dividing these vessels has been based on the outcomes from uncontrolled case series of patients undergoing Nissen fundoplication either with vs. without division of the short gastric vessels. However, the results from these studies have been conflicting, with different proponents reporting good results irrespective of whether these vessels have been divided or not. To address this issue, six randomized trials that enrolled a total of 438 patients have been reported. None of these trials demonstrated any differences for the postoperative dysphagia or recurrent gastro-esophageal reflux. However, in the three largest of the six trials an increased incidence of flatulence and bloating symptoms, as well as greater difficulty with belching, was seen in patients in whom the short gastric vessels were divided. A recent meta-analysis from Engstrom et al, generated by combining the raw data from Australian and Swedish trials, evaluated a larger cohort of 201 patients, with 12 years of follow-up in 170, and also confirmed equivalent reflux control but found more abdominal bloating after division of the short gastric vessels. Overall, these trials fail to support the belief that dividing the short gastric vessels improves any outcome following Nissen fundoplication. The trials actually suggest that dividing the vessels increases the complexity of the procedure and leads to a poorer outcome due to the increase in bloating symptoms. Nissen vs. Posterior Partial Fundoplication Eleven randomized trials have compared Nissen vs. posterior partial fundoplication. Some of the trials contributed little to the pool of evidence, as they are either small or underpowered, and failed to show significant outcome differences. The larger trials, however, have consistently demonstrated equivalent reflux control, but they also show a reduced incidence of wind-related side-effects (flatulence, bloating, and inability to belch) following posterior partial fundoplication procedures, although less dysphagia following a posterior fundoplication was only demonstrated in 2 of the 11 trials. Lundell et al reported the outcomes of Nissen vs. Toupet partial fundoplication in a trial that enrolled 137 patients with reported follow-up to 18 years. Reflux control and dysphagia symptoms were similar, but flatulence was commoner after Nissen fundoplication at some medium-term follow-up time points, and revision surgery was more common following Nissen fundoplication, mainly to correct postoperative paraoesophageal herniation. At 18 years follow-up, success rates of more than 80% were reported for both procedures, as well as no significant differences in the incidence of side effects. The data from this trial suggested that the mechanical side effects following Nissen fundoplication progressively improve with very long-term follow-up. Strate et al reported 2-year follow-up in a trial that enrolled 200 patients. Approximately 85% of each group was satisfied with the clinical outcome, but dysphagia was significantly more common following Nissen fundoplication (19 vs. 8 patients). Other trials (Guérin et al–140 patients, Booth et al–127, Khan et al–121, Shaw et al–100) also report similar reflux control within the first few years of follow-up. Only Booth et al demonstrated less dysphagia following posterior fundoplication. Subgroup analysis in 3 trials (Booth, Shaw, Zornig) did not reveal differences between patients with vs. without poor preoperative oesophageal motility. Overall these trials suggest that some side-effects, mainly wind-related issues, are less common following posterior partial fundoplication. However, the hypothesis that dysphagia is less of a problem following posterior partial fundoplication has only been substantiated in 2 of 11 trials. Nissen vs. Anterior Fundoplication Six trials have evaluated Nissen vs. anterior partial fundoplication variants. Four have assessed Nissen vs. anterior 180° partial fundoplication (Watson et al–107 patients, Baigrie et al–161, Cao et al–100, Raue et al–64). These trials all demonstrated equivalent reflux control, but less dysphagia and less wind-related side effects after anterior 180° partial fundoplication at up to 5 years follow-up. Only the study from Watson et al has reported follow-up to 10 years, and at late follow-up in their trial there were no significant outcome differences for the two procedures, with equivalent control of reflux, and no differences for side effects due to a progressive decline in dysphagia as follow-up extended beyond 5 years. Two trials compared laparoscopic anterior 90° partial fundoplication vs. Nissen fundoplication (Watson et al–112 patients, Spence et al–79). In both of these trials, side-effects were less common following anterior 90° fundoplication, but this was offset by a slightly higher incidence of recurrent reflux at up to 5 years follow-up. Satisfaction with the overall outcome was similar for both fundoplication variants. Anterior vs. Posterior Partial Fundoplication Two randomized trials have directly compared anterior vs. posterior partial fundoplication. Hagedorn et al randomized 95 patients to undergo either Toupet vs. anterior 120° partial fundoplication, and Khan et al enrolled 103 patients to anterior 180° vs. posterior partial fundoplication. Both studies demonstrated better reflux control, offset by more side effects following posterior partial fundoplication. The anterior 120° partial fundoplication performed by Hagedorn et al was similar to the anterior 90° variant described above. However, the outcomes following this procedure were much worse in this trial than the outcomes in other studies, with the average exposure time to acid (pH <4%–5.6%) following anterior fundoplication in their study unusually high compared to other studies. Khan et al only reported 6 months follow-up, and longer-term outcomes are awaited before drawing firm conclusions. The overall results from all eight trials that included an anterior fundoplication variant suggest that this type of fundoplication achieves satisfactory reflux control, with less dysphagia and other side-effects, yielding a good overall outcome. However, the reduced incidence of troublesome sideeffects is traded off against a higher risk of recurrent reflux. Outcome of Antireflux Surgery in Patients With Barrett’s Esophagus. Few studies have focused on the alleviation of symptoms after antireflux surgery in patients with BE (Table 25-7). Those that are available document excellent to good results in 72% to 95% of patients at 5 years following surgery. Several nonrandomized studies have compared medical and surgical therapy and report better outcomes after antireflux surgery. Parrilla and colleagues reported the only randomized trial to evaluate this issue. They enrolled 101 patients over 18 years, and median follow-up was 6 years. Medical therapy consisted of 20 mg of omeprazole (PPI) twice daily since 1992 in all medically treated patients, and surgical therapy consisted of an open Nissen Reoperation for Failed Antireflux Repairs. Failure of an Table 25-7 Symptomatic outcome of surgical therapy for Barrett’s esophagus % EXCELLENT MEAN TO GOOD FOLLOW-UP, RESPONSE YEARS YEAR Starnes 1984 8 75 2 Williamson 1990 37 92 3 DeMeester 1990 35 77 3 McDonald 1996 113 82.2 6.5 Ortiz 1996 32 90.6 5 fundoplication. The symptomatic outcome in the two groups was nearly identical, although esophagitis and/or stricture persisted in 20% of the medically treated patients, compared to only 3% to 7% of patients following antireflux surgery. About 15% of patients had abnormal acid exposure after surgery. Although pH data were not routinely collected in patients on PPI therapy, in the subgroup of 12 patients that did have 24-hour monitoring on treatment, 3 of 12 (25%) had persistently high esophageal acid exposure, and most (75%) had persistently high bilirubin exposure. The common belief that Barrett’s epithelium cannot be reversed by antireflux surgery may not be correct. Within the control arm of a randomized trial of ablation vs. surveillance, Bright and associates identified approximately 50% regression in the length of Barrett’s esophagus in 20 patients within the control arm of a randomized trial of ablation vs. surveillance. Current data indicate that patients with BE should remain in an endoscopic surveillance program following antireflux surgery. Biopsy specimens should be reviewed by a pathologist with expertise in the field. If low-grade dysplasia is confirmed, biopsy specimens should be repeated after 12 weeks of high-dose acid suppression therapy. If high-grade dysplasia or intramucosal cancer is evident on more than one biopsy specimen, then treatment is escalated. Treatment options include endoscopic mucosal resection, endoscopic ablation of the BE, or esophageal resection. Esophageal resection is advisable when an invasive cancer (stage T1b or deeper) is present, or for multifocal long segment BE in younger and fit patients in whom endoscopic treatments are unlikely to be adequate. Endoscopic mucosal resection allows smaller intramucosal tumors to be removed with clear pathology margins, and it can be used as a “big biopsy” to obtain better pathological staging, and even to excise shorter segments of BE in a piecemeal fashion. Ablation, commonly using radiofrequency ablation, has been shown at short-term follow-up in a randomized trial to reduce the rate of progression from high grade dysplasia to invasive cancer by approximately 50%. However, following any endoscopic treatment, patients need to continue with close endoscopic surveillance as recurrence can occur and the longer-term outcome following these treatments remains uncertain. Early detection and treatment have been shown to decrease the mortality rate from esophageal cancer in these patients. If the dysplasia is reported as lower grade or indeterminant, then inflammatory change that is often confused with dysplasia should be suppressed by a course of acid suppression therapy in high doses for 2 to 3 months, followed by rebiopsy of the Barrett’s segment. GIANT DIAPHRAGMATIC (HIATAL) HERNIAS With the advent of clinical radiology, it became evident that a diaphragmatic hernia was a relatively common abnormality and was not always accompanied by symptoms. Three types of esophageal hiatal hernia were identified: (a) the sliding hernia, type I, characterized by an upward dislocation of the cardia in the posterior mediastinum (Fig. 25-39A); (b) the rolling or PEH, type II, characterized by an upward dislocation of the gastric fundus alongside a normally positioned cardia (Fig. 25-39B); and (c) the combined sliding-rolling or mixed hernia, type III, characterized by an upward dislocation of both the cardia and the gastric fundus (Fig. 25-39C). The end stage of type I and type II hernias occurs when the whole stomach migrates up into the chest by rotating 180° around its longitudinal axis, with the cardia and pylorus as fixed points. In this situation, the abnormality is usually referred to as an intrathoracic stomach (Fig. 25-39D). In some taxonomies, a type IV hiatal hernia is declared when an additional organ, usually the colon, herniates as well. Types II–IV hiatal hernias are also referred to as paraesophageal hernia (PEH), as a portion of the stomach is situated adjacent to the esophagus, above the gastroesophageal junction. Incidence and Etiology The true incidence of a hiatal hernia is difficult to determine because of the absence of symptoms in a large number of patients who are subsequently shown to have a hernia. When radiographic examinations are done in response to GI symptoms, 1045 CHAPTER 25 ESOPHAGUS AND DIAPHRAGMATIC HERNIA AUTHOR NO. OF PATIENTS antireflux procedure occurs when, after the repair, the patient is unable to swallow normally, experiences upper abdominal discomfort during and after meals, or has recurrence or persistence of reflux symptoms. The assessment of these symptoms and the selection of patients who need further surgery are challenging problems. Functional assessment of patients who have recurrent, persistent, or emergent new symptoms following a primary antireflux repair is critical to identifying the cause of the failure. Analysis of patients requiring reoperation after a previous antireflux procedure shows that placement of the wrap around the stomach is the most frequent cause for failure after open procedures, while herniation of the repair into the chest is the most frequent cause of failure after a laparoscopic procedure. Partial or complete breakdown of the fundoplication and construction of a too-tight a fundoplication or overnarrowing the esophageal hiatus occurs with both open and closed procedures. Patients who have recurrence of heartburn and regurgitation without dysphagia and have good esophageal motility are most amenable to reoperation, and they can be expected to have an excellent outcome. When dysphagia is the cause of failure, the situation can be more difficult to manage. If the dysphagia occurred immediately following the repair, it is usually due to a technical failure, most commonly a misplaced fundoplication around the upper stomach, or overnarrowing of the esophageal diaphragmatic hiatus and reoperation is usually satisfactory. When dysphagia is associated with poor motility and multiple previous repairs, further revision fundoplication is unlikely to be successful, and in otherwise fit patients it is appropriate to seriously consider esophageal resection. With each reoperation, the esophagus is damaged further, and the chance of preserving function is decreased. Also, blood supply is reduced, and ischemic necrosis of the esophagus can occur after several previous mobilizations. 1046 PART II SPECIFIC CONSIDERATIONS A B C D Figure 25-39. A. Radiogram of a type I (sliding) hiatal hernia. B. Radiogram of a type II (rolling or paraesophageal) hernia. C. Radiogram of a type III (combined sliding-rolling or mixed) hernia. D. Radiogram of an intrathoracic stomach. This is the end stage of a large hiatal hernia regardless of its initial classification. Note that the stomach has rotated 180° around its longitudinal axis, with the cardia and pylorus as fixed points. (Reproduced with permission from Nyhus LM, Condon RE: Hernia, 3rd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 1989.) Clinical Manifestations The clinical presentation of a giant hiatal (paraesophageal) hernia differs from that of a sliding hernia. There is usually a higher prevalence of symptoms of dysphagia and postprandial fullness with PEHs, but the typical symptoms of heartburn and regurgitation present in sliding hiatal hernias can also occur. Both are caused by gastroesophageal reflux secondary to an underlying mechanical deficiency of the cardia. The symptoms of dysphagia and postprandial fullness in patients with a PEH are explained by the compression of the adjacent esophagus by a distended cardia, or twisting of the GEJ by the torsion of the stomach that occurs as it becomes progressively displaced in the chest. The postprandial fullness or retrosternal chest pain is a thought to be a result of distension of the stomach with gas or food in the hiatal hernia. Many patients with sliding hernias and reflux symptoms will lose the reflux symptoms when the hernia evolves into the paraesophageal variety. This can be explained by the recreation of the cardiophrenic angle when the stomach herniates alongside the GEJ or becomes twisted in the sac. Repair of the hernia without addressing the reflux can create extremely bothersome heartburn. Respiratory complications are frequently associated with a PEH and consist of dyspnea and recurrent pneumonia from aspiration. New research demonstrates that the cause of dyspnea in the presence of a giant PEH is more likely to be left atrial compression, decreasing cardiac output, than a restrictive pulmonary effect, as has been hypothesized for many years. Approximately one-third of patients with a PEH are found to be anemic, which is due to recurrent bleeding from ulceration of the gastric mucosa in the herniated portion of the stomach, even if ulcerations are not detected at the time of endoscopy. The association of anemia and PEH is best proven by fixing the hernia. Anemia is corrected in >90% of patients with this condition. With time, more and more stomach migrates into the chest and can cause intermittent foregut obstruction due to the rotation that has occurred. In contrast, many patients with PEH are asymptomatic or complain of minor symptoms. However, the presence of a PEH can be life-threatening in that the hernia can lead to sudden catastrophic events, such as excessive bleeding or volvulus with acute gastric obstruction or infarction. With mild dilatation of the stomach, the gastric blood supply can be markedly reduced, causing gastric ischemia, ulceration, perforation, and sepsis. The probability of incarceration/strangulation is not well known, although recent studies suggest that the lifetime risk is less than 5%, making this concern an insufficient concern for routine repair of the asymptomatic PEH. The symptoms of sliding hiatal hernias are usually due to functional abnormalities associated with gastroesophageal reflux and include heartburn, regurgitation, and dysphagia. These patients have a mechanically defective LES, giving rise to the reflux of gastric juice into the esophagus and the symptoms of heartburn and regurgitation. The symptom of dysphagia occurs from the presence of mucosal edema, Schatzki’s ring, stricture, or the inability to organize peristaltic activity in the body of the esophagus as a consequence of the disease. There is a group of patients with sliding hiatal hernias not associated with reflux disease who have dysphagia without any obvious endoscopic or manometric explanation. Video barium radiograms have shown that the cause of dysphagia in these patients is an obstruction of the swallowed bolus by diaphragmatic impingement on the herniated stomach. Manometrically, this is reflected by a double-humped high-pressure zone at the GEJ. The first pressure rise is due to diaphragmatic impingement on the herniated stomach, and the second is due to the true distal esophageal sphincter. These patients usually have a mechanically competent sphincter, but the impingement of the diaphragm on the stomach can result in propelling the contents of the supradiaphragmatic portion of the stomach up into the esophagus and pharynx, resulting in complaints of pharyngeal regurgitation and aspiration. Consequently, this abnormality is often confused with typical GERD. Surgical reduction of the hernia results in relief of the dysphagia in 91% of patients. Diagnosis A chest X-ray with the patient in the upright position can diagnose a hiatal hernia if it shows an air-fluid level behind the cardiac shadow. This is usually caused by a PEH or an intrathoracic stomach. The accuracy of the upper GI barium study in detecting a paraesophageal hiatal hernia is greater than for a sliding hernia because the latter can often spontaneously reduce. The paraesophageal hiatal hernia is a permanent herniation of the stomach into the thoracic cavity, so a barium swallow provides the diagnosis in virtually every case. Attention should be focused on the position of the GEJ, when seen, to differentiate it from a type II hernia (see Fig. 25-39B and C). Fiber-optic esophagoscopy is useful in the diagnosis and classification of a hiatal hernia because the scope can be retroflexed. In this position, a sliding hiatal hernia can be identified by noting a gastric pouch lined with rugal folds extending above the impression caused by the crura of the diaphragm, or measuring at least 2 cm between the crura, identified by having the patient sniff, and the squamocolumnar junction on withdrawal of the scope (Fig. 25-40). A PEH is identified on retroversion of the scope by noting a separate orifice adjacent to the GEJ into which gastric rugal folds ascend. A sliding-rolling or mixed hernia can be identified by noting a gastric pouch lined with rugal folds above the diaphragm, with the GEJ entering about midway up the side of the pouch. 1047 CHAPTER 25 ESOPHAGUS AND DIAPHRAGMATIC HERNIA the incidence of a sliding hiatal hernia is seven times higher than that of a PEH. The PEH is also known as the giant hiatal hernia. Over time the pressure gradient between the abdomen and chest enlarges the hiatal hernia. In many cases the type 1 sliding hernia will evolve into a type III mixed hernia. Type II hernias are quite rare. The age distribution of patients with PEHs is significantly different from that observed in sliding hiatal hernias. The median age of the former is 61 years old; of the latter, 48 years old. PEHs are more likely to occur in women by a ratio of 4:1. Structural deterioration of the phrenoesophageal membrane over time may explain the higher incidence of hiatal hernias in the older age group. These changes involve thinning of the upper fascial layer of the phrenoesophageal membrane (i.e., the supradiaphragmatic continuation of the endothoracic fascia) and loss of elasticity in the lower fascial layer (i.e., the infradiaphragmatic continuation of the transversalis fascia). Consequently, the phrenoesophageal membrane yields to stretching in the cranial direction due to the persistent intra-abdominal pressure and the tug of esophageal shortening on swallowing. Interestingly, the stretching and thinning occurs more anteriorly and posteriorly, with fixation of the left crus of the diaphragm to the stomach at the 3 o’clock position, as viewed from the foot. This creates an anterior and posterior hernia sac, the latter of which is often filled with epiphrenic and retroperitoneal fat. These observations point to the conclusion that the development of a hiatal hernia is an age-related phenomenon secondary to repetitive upward stretching of the phrenoesophageal membrane. 1048 PART II SPECIFIC CONSIDERATIONS Figure 25-40. Endoscopic view through a retroflexed fiber-optic gastroscope showing the shaft of the scope (arrow) coming down through a sliding hernia. Note the gastric rugal folds extending above the impression caused by the crura of the diaphragm. (Reproduced with permission from Nyhus LM, Condon RE: Hernia, 3rd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 1989.) Pathophysiology Physiologic testing with 24-hour esophageal pH monitoring has shown increased esophageal exposure to acid gastric juice in 60% of the patients with a paraesophageal hiatal hernia, compared with the observed 71% incidence in patients with a sliding hiatal hernia. It is now recognized that paraesophageal hiatal hernia can be associated with pathologic gastroesophageal reflux. Physiologic studies have also shown that the competency of the cardia depends on an interrelationship between distal esophageal sphincter pressure, the length of the sphincter that is exposed to the positive-pressure environment of the abdomen, and the overall length of the sphincter. A deficiency in any one of these manometric characteristics of the sphincter is associated with incompetency of the cardia regardless of whether a hernia is present. Patients with a PEH who have an incompetent cardia have been shown to have a distal esophageal sphincter with normal pressure, but a shortened overall length and displacement outside the positive-pressure environment of the abdomen. One might expect esophageal body function to be diminished with the esophagus “accordioned” up into the chest. Surprisingly, esophageal peristalsis in patients with PEH is normal in 88%. Treatment The treatment of paraesophageal hiatal hernia is largely surgical. Controversial aspects include: (a) indications for repair, (b) diaphragmatic repair, (c) role of fundoplication, and (d) existence and treatment of the short esophagus. Indications and Surgical Approach. The presence of a paraesophageal hiatal hernia has traditionally been considered an indication for surgical repair. This recommendation is largely based upon two clinical observations. First, retrospective studies have shown a significant incidence of catastrophic, life-threatening complications of bleeding, infarction, and perforation in patients being followed with known paraesophageal herniation. Second, emergency repair carries a high mortality. In the classic report of Skinner and Belsey, six of 21 patients with a PEH, treated medically because of minimal symptoms, died from the complications of strangulation, perforation, exsanguinating hemorrhage, or acute dilatation of the herniated intrathoracic stomach. For the most part, these catastrophes occurred without warning. Others have reported similar findings. Recent studies suggest that catastrophic complications may be somewhat less common. Allen and colleagues followed 23 patients for a median of 78 months with only four patients progressively worsening. There was a single mortality secondary to aspiration that occurred during a barium swallow examination to investigate progressive symptoms. Although emergency repairs had a median hospital stay of 48 days compared to a stay of 9 days in those having elective repair, there were only three cases of gastric strangulation in 735 patient-years of follow-up. If surgery is delayed and repair is done on an emergency basis, operative mortality is high, compared to <1% for an elective repair. With this in mind, patients with a PEH are generally counseled to have elective repair of their hernia, particularly if they are symptomatic. Watchful waiting of asymptomatic PEHs may be an acceptable option. The surgical approach to repair of a paraesophageal hiatal hernia may be either transabdominal (laparoscopic or open) or Each has its advantages and disadvantages. A 4 transthoracic. transthoracic approach facilitates complete esophageal mobilization but is rarely used because the access trauma and postoperative pain are significantly greater than a laparoscopic approach. The transabdominal approach facilitates reduction of the volvulus that is often associated with PEHs. Although some degree of esophageal mobilization can be accomplished transhiatally, complete mobilization to the aortic arch is difficult or impossible without risk of injury to the vagal nerves. Laparoscopic repair of PEH would appear to have become the standard approach. Laparoscopic repair of a pure type II, or mixed type III PEH is an order of magnitude more difficult than a standard laparoscopic Nissen fundoplication. Most would recommend that these procedures are best avoided until the surgeon has accumulated considerable experience with laparoscopic antireflux surgery. There are several reasons for this. First, the vertical and horizontal volvulus of the stomach often associated with PEHs makes identification of the anatomy, in particular the location of the esophagus, difficult. Second, dissection of a large PEH sac may result in significant bleeding if the surgeon deviates from the correct plane of dissection between the peritoneal sac and the endothoracic fascia. Finally, redundant tissue present at the GEJ following dissection of the sac frustrates the creation of a fundoplication. This tissue, which includes the epiphrenic fat pad and hernia sac should be removed at the time of PEH repair. Mindful of these difficulties, and given appropriate experience, patients with PEH may be approached laparoscopically, with expectation of success in the majority. Diaphragmatic Repair It has been shown that PEH repair has a relatively high incidence of recurrence (10–40%) when the crura is closed primarily with permanent suture. Techniques to reduce hernia recurrence continue to evolve. Most surgeons believe that recurrence may be reduced with the use of synthetic or biologic mesh to reinforce the standard crural closure. Randomized controlled studies have demonstrated a reduction in PEH recurrence rate when mesh was used. Nonabsorbable synthetic mesh must be used carefully and not in a keyhole fashion at the hiatus because of a potential risk of esophagus or gastric erosion and mesh infection. Biologic mesh (acellular porcine dermis, acellular human dermis, porcine small intestinal submucosa) has become more widely used, but these meshes are significantly more expensive than synthetic mesh, and the only randomized study supporting biologic mesh usage failed to demonstrate superiority over suture alone after 5 years of rigorous follow-up. Controversy remains as to whether to perform an antireflux procedure at all, in selected cases only, or in all patients. Most advocate the routine addition of an antireflux procedure following repair of the hernia defect. There are several reasons for this. Physiologic testing with 24-hour esophageal pH monitoring has shown increased esophageal exposure to acid gastric juice in 60% to 70% of patients with a paraesophageal hiatal hernia, nearly identical to the observed 71% incidence in patients with a sliding hiatal hernia. Furthermore, there is no relation between the symptoms experienced by the patient with a PEH and the competency of the cardia. Finally, dissection of the gastroesophageal esophagus may lead to postoperative reflux despite a negative preoperative pH score. The Short Esophagus and PEH Giant PEH can be associated with a short esophagus in up to 5% to 20% of patients as a result of chronic cephalad displacement of the GEJ. The presence of a short esophagus increases the difficulty of laparoscopic PEH repair. Approximately 10% to 20% of surgical failures with PEH repair is due to the lack of recognition of a short esophagus. Preoperative results of barium swallow and esophagogastroduodenoscopy may provide an indication of short esophagus, but no combination of preoperative clinical variables reliably predict the presence of short esophagus, defined as the failure to achieve 2.5 cm of intra-abdominal esophagus with standard mediastinal dissection techniques. Hence, the diagnosis of this entity continues to be made definitively only in the operating room. Collis gastroplasty achieves esophageal lengthening by creation of a neoesophagus using the gastric cardia. The totally laparoscopic approach to the short esophagus has evolved from a method using an end-to-end anastomosis circular stapler to the current approach that uses a linear stapler creating a stapled wedge gastroplasty. Elements of importance in fashioning the fundoplication after Collis gastroplasty include placement of the initial suture of the fundoplication on the esophagus, immediately above the GEJ to ensure that acid-secreting (gastric) mucosa does not reside above the fundoplication. A second element that ensures safety and avoids wrap deformation is to place the gastric portion of the staple line against the neoesophagus, such that the tip of the gastric staple line sits adjacent to the middle suture of the fundoplication on the right side of the esophagus. SCHATZKI’S RING Schatzki’s ring is a thin submucosal circumferential ring in the lower esophagus at the squamocolumnar junction, often associated with a hiatal hernia. Its significance and pathogenesis are unclear (Fig. 25-41). The ring was first noted by Templeton, but Schatzki and Gary defined it as a distinct entity in 1953. Its prevalence varies from 0.2% to 14% in the general population, depending on the technique of diagnosis and the criteria used. Stiennon believed the ring to be a pleat of mucosa formed by infolding of redundant esophageal mucosa due to shortening of the esophagus. Others believe the ring to be congenital, and still others suggest it is an early stricture resulting from inflammation of the esophageal mucosa caused by chronic reflux. Schatzki’s ring is a distinct clinical entity having different symptoms, upper GI function studies, and response to treatment compared with patients with a hiatal hernia, but without a ring. Twenty-four-hour esophageal pH monitoring has shown that patients with a Schatzki’s ring have a lower incidence of reflux than hiatal hernia controls. They also have better LES function. This, together with the presence of a ring, could represent a protective mechanism to prevent gastroesophageal reflux. Results Most outcome studies report relief of symptoms following surgical repair of PEHs in more than 90% of patients. The current literature suggests that laparoscopic repair of a paraesophageal hiatal hernia can be successful. Most authors report symptomatic improvement in 80% to 90% of patients, and <10% to 15% prevalence of recurrent symptomatic hernia. However, the problem of recurrent asymptomatic or minimally symptomatic hernia following PEH repair, open or laparoscopic, is Figure 25-41. Barium esophagogram showing Schatzki’s ring (i.e., a thin circumferential ring in the distal esophagus at the squamocolumnar junction). Below the ring is a hiatal hernia. 1049 CHAPTER 25 ESOPHAGUS AND DIAPHRAGMATIC HERNIA Role of Fundoplication in Giant Hiatal Hernia Repair. becoming increasingly appreciated. Recurrent hiatal hernia is the most common cause of anatomic failure following laparoscopic Nissen fundoplication done for GERD (5–10%), but this risk is compounded for the giant hernia where radiologic recurrence is detected in 25% to 40% of patients. It appears that optimal results with open or laparoscopic giant hiatal hernia repair should include options for mesh buttressing of hiatal closure and selective esophageal lengthening with one of the many techniques developed for the creation of a Collis gastroplasty. Despite this high incidence of radiologic recurrence, and the surgical pursuit of a remedy, it must be reinforced that asymptomatic recurrent hernias, like primary PEH, do not need to be repaired. The risk of incarceration, strangulation, or obstruction is minimal. 1050 PART II SPECIFIC CONSIDERATIONS Symptoms associated with Schatzki’s ring are brief episodes of dysphagia during hurried ingestion of solid foods. Its treatment has varied from dilation alone to dilation with antireflux measures, antireflux procedure alone, incision, and even excision of the ring. Little is known about the natural progression of Schatzki’s rings. Using radiologic techniques, Chen and colleagues showed progressive stenosis of rings in 59% of patients, whereas Schatzki found that the rings decreased in diameter in 29% of patients and remained unchanged in the rest. Symptoms in patients with a ring are caused more by the presence of the ring than by gastroesophageal reflux. Most patients with a ring but without proven reflux respond to one dilation, while most patients with proven reflux require repeated dilations. In this regard, the majority of Schatzki’s ring patients without proven reflux have a history of ingestion of drugs known to be damaging to the esophageal mucosa. Bonavina and associates have suggested drug-induced injury as the cause of stenosis in patients with a ring, but without a history of reflux. Because rings also occur in patients with proven reflux, it is likely that gastroesophageal reflux also plays a part. This is supported by the fact that there is less drug ingestion in the history of these patients. Schatzki’s ring is probably an acquired lesion that can lead to stenosis from chemicalinduced injury by pill lodgment in the distal esophagus, or from reflux-induced injury to the lower esophageal mucosa. The best form of treatment of a symptomatic Schatzki’s ring in patients who do not have reflux consists of esophageal dilation for relief of the obstructive symptoms. In patients with a ring who have proven reflux and a mechanically defective sphincter, an antireflux procedure is necessary to obtain relief and avoid repeated dilation. SCLERODERMA Scleroderma is a systemic disease accompanied by esophageal abnormalities in approximately 80% of patients. In most, the disease follows a prolonged course. Renal involvement occurs in a small percentage of patients and signals a poor prognosis. The onset of the disease is usually in the third or fourth decade of life, occurring twice as frequently in women as in men. Small vessel inflammation appears to be an initiating event, with subsequent perivascular deposition of normal collagen, which may lead to vascular compromise. In the GI tract, the predominant feature is smooth muscle atrophy. Whether the atrophy in the esophageal musculature is a primary effect or occurs secondary to a neurogenic disorder is unknown. The results of pharmacologic and hormonal manipulation, with agents that act either indirectly via neural mechanisms or directly on the muscle, suggest that scleroderma is a primary neurogenic disorder. Methacholine, which acts directly on smooth muscle receptors, causes a similar increase in LES pressure in normal controls and in patients with scleroderma. Edrophonium, a cholinesterase inhibitor that enhances the effect of acetylcholine when given to patients with scleroderma, causes an increase in LES pressure that is less marked in these patients than in normal controls, suggesting a neurogenic rather than myogenic etiology. Muscle ischemia due to perivascular compression has been suggested as a possible mechanism for the motility abnormality in scleroderma. Others have observed that in the early stage of the disease, the manometric abnormalities may be reversed by reserpine, an agent that depletes catecholamines from the adrenergic system. This suggests that, in early scleroderma, an adrenergic overactivity may be present that causes a parasympathetic inhibition, supporting Scleroderma Esophagus 25 cm S mmHg S S S 35 – 0 35 – Esophagus 30 cm 0 35 – Esophagus 35 cm 0 Figure 25-42. Esophageal motility record in a patient with scleroderma showing aperistalsis in the distal two-thirds of the esophageal body with peristalsis in the proximal portion. (Reproduced with permission from Waters PF, DeMeester TR: Foregut motor disorders and their surgical management, Med Clin North Am. 1981 Nov;65(6):1235-1268.) a neurogenic mechanism for the disease. In advanced disease manifested by smooth muscle atrophy and collagen deposition, reserpine no longer produces this reversal. Consequently, from a clinical perspective, the patient can be described as having a poor esophageal pump and a poor valve. The diagnosis of scleroderma can be made manometrically by the observation of normal peristalsis in the proximal striated esophagus, with absent peristalsis in the distal smooth muscle portion (Fig. 25-42). The LES pressure is progressively weakened as the disease advances. Because many of the systemic sequelae of the disease may be nondiagnostic, the motility pattern is frequently used as a specific diagnostic indicator. Gastroesophageal reflux commonly occurs in patients with scleroderma because they have both hypotensive sphincters and poor esophageal clearance. This combined defect can lead to severe esophagitis and stricture formation. The typical barium swallow shows a dilated, bariumfilled esophagus, stomach, and duodenum, or a hiatal hernia with distal esophageal stricture and proximal dilatation (Fig. 25-43). Traditionally, esophageal symptoms have been treated with PPIs, antacids, elevation of the head of the bed, and multiple dilations for strictures, with generally unsatisfactory results. The degree of esophagitis is usually severe and may lead to marked esophageal shortening as well as stricture. Scleroderma patients have frequently had numerous dilations before they are referred to the surgeon. The surgical management is somewhat controversial, but the majority of opinion suggests that a partial fundoplication (anterior or posterior) performed laparoscopically is the procedure of choice. The need for a partial fundoplication is dictated by the likelihood of severe dysphagia if a total fundoplication is performed in the presence of aperistalsis. Esophageal shortening may require a Collis gastroplasty in combination with a partial fundoplication. Surgery reduces esophageal acid exposure but does not return it to normal because of the poor EOSINOPHILIC ESOPHAGITIS 1051 Eosinophilic esophagitis (EE) was first described in 1977, but it has become well known only in the last two decades. The condition is characterized by a constellation of symptoms, endoscopic and radiologic findings, and distinctive pathology. The etiology of eosinophilic esophagitis is not entirely known but its similarities, immunologically, to asthma suggest that it is a form of “allergic esophagitis.” The presentation of eosinophilic esophagitis is chest pain (often postprandial) and dysphagia. Dysphagia may occur with liquids or solids, but solid food dysphagia is most common. Because dysphagia and chest pain are characteristic of GERD, EE is often confused with GERD; however, EE does not respond to proton pump inhibitors. The evaluation of the patient with EE and dysphagia and chest pain with esophagram and endoscopy usually reveals the diagnosis. Signs A barium swallow should be the first test obtained in the patient with dysphagia. EE has a characteristic finding often called the “ringed esophagus” or the “feline esophagus,” as the esophageal rings are felt to look like the stripes on a housecat (Fig. 25-44). The endoscopic appearance of EE is also characteristic, and also appears as a series of rings (Fig. 25-45). Pathology Figure 25-43. Barium esophagogram of a patient with scleroderma and stricture. Note the markedly dilated esophagus and retained food material. (Reproduced with permission from Waters PF, DeMeester TR: Foregut motor disorders and their surgical management, Med Clin North Am. 1981 Nov;65(6):1235-1268.) clearance function of the body of the esophagus. Only 50% of the patients have a good-to-excellent result. If the esophagitis is severe, or there has been a previous failed antireflux procedure and the disease is associated with delayed gastric emptying, a gastric resection with Roux-en-Y gastrojejunostomy has proved the best option. Endoscopic biopsy specimens should be taken when eosinophilic esophagus is suspected. To make the diagnosis of EE, the pathologist should see a minimum of 15 eosinophils per high powered field, usually at the base of the epithelium (Fig. 25-46). Treatment The treatment of EE is largely symptomatic and includes testing for food allergies and elimination of identified items from the diet. Second-line therapy includes inhaled or ingested corticosteroids, as would be used to treat asthma. If dysphagia is not relieved with steroids, it may be necessary to dilate the Figure 25-44. The esophagus on the left shows a stacking of rings, demonstrating eosinophilic esophagus. The esophagus on the right is a normal barium swallow. CHAPTER 25 ESOPHAGUS AND DIAPHRAGMATIC HERNIA Symptoms 1052 the meal, is the last to finish, or is forced to interrupt or avoid a social meal; and whether he or she has been admitted to the hospital for food impaction. These assessments, plus an evaluation of the patient’s nutritional status, help to determine how severe the dysphagia is and judge the need for surgical intervention, rather than more conservative methods of treating dysphagia. Motility Disorders of the Pharynx and Upper Esophagus—Transit Dysphagia PART II SPECIFIC CONSIDERATIONS Figure 25-45. The endoscopic appearance of eosinophilic esophagitis is characteristically a series of stacked mucosal rings. esophagus. Because of the length of esophageal involvement, rigid dilators (Maloney or Savary) are often used. Great care must be exercised, as the inflamed EE is quite friable. The mucosal tears easily, and esophageal perforation (full thickness laceration) has been reported with EE dilation. MOTILITY DISORDERS OF THE PHARYNX AND ESOPHAGUS Clinical Manifestations Dysphagia (i.e., difficulty in swallowing) is the primary symptom of esophageal motor disorders. Its perception by the patient is a balance between the severity of the underlying abnormality causing the dysphagia and the adjustment made by the patient in altering eating habits. Consequently, any complaint of dysphagia must include an assessment of the patient’s dietary history. It must be known whether the patient experiences pain, chokes, or vomits with eating; whether the patient requires liquids with Disorders of the pharyngeal phase of swallowing result from a discoordination of the neuromuscular events involved in chewing, initiation of swallowing, and propulsion of the material from the oropharynx into the cervical esophagus. They can be categorized into one or a combination of the following abnormalities: (a) inadequate oropharyngeal bolus transport; (b) inability to pressurize the pharynx; (c) inability to elevate the larynx; (d) discoordination of pharyngeal contraction and cricopharyngeal relaxation; and (e) decreased compliance of the pharyngoesophageal segment secondary to neuromuscular disease. The latter may result in incomplete relaxation of the cricopharyngeus and cervical esophagus during swallowing. Taken together, these disorders are termed transit dysphagia by many. Transit dysphagia is usually congenital or results from acquired disease involving the central and peripheral nervous system. This includes cerebrovascular accidents, brain stem tumors, poliomyelitis, multiple sclerosis, Parkinson’s disease, pseudobulbar palsy, peripheral neuropathy, and operative damage to the cranial nerves involved in swallowing. Pure muscular diseases such as radiation-induced myopathy, dermatomyositis, myotonic dystrophy, and myasthenia gravis are less common causes. Rarely, extrinsic compression of the cervical esophagus by thyromegaly, lymphadenopathy, or hyperostosis of the cervical spine can cause transit dysphagia. Diagnostic Assessment of the Cricopharyngeal Segment Transit dysphagia difficult to assess with standard manometric techniques because of the rapidity of the oropharyngeal phase of swallowing, the elevation of the larynx, and the asymmetry of the cricopharyngeus. Video- or cineradiography is currently the Figure 25-46. A cluster of eosinophils are visualized in the esophageal epithelium in a patient with EE. Time 0 Peak pharyngeal pressure 1053 Atmospheric pressure Bolus pressure final initial A B Figure 25-47. A. Zenker’s diverticulum, initially discovered 15 years ago and left untreated. B. Note its marked enlargement and evidence of laryngeal inlet aspiration on recent esophagogram. (Reproduced with permission from Waters PF, DeMeester TR: Foregut motor disorders and their surgical management, Med Clin North Am. 1981 Nov;65(6):1235-1268.) Maximum residual (MaxR) contraction B0 Minimum Residual (MinR) Subatomic pressure B most objective test to evaluate oropharyngeal bolus transport, pharyngeal compression, relaxation of the pharyngoesophageal segment, and the dynamics of airway protection during swallowing. It readily identifies a diverticulum (Fig. 25-47), stasis of the contrast medium in the valleculae, a cricopharyngeal bar, and/or narrowing of the pharyngoesophageal segment. These are anatomic manifestations of neuromuscular disease, and they result from the loss of muscle compliance in portions of the pharynx and esophagus composed of skeletal muscle. Careful analysis of video- or cineradiographic studies combined with manometry using specially designed catheters can identify the cause of a pharyngoesophageal dysfunction in most situations (Fig. 25-48). Motility studies may demonstrate inadequate pharyngeal pressurization, insufficient or lack of cricopharyngeal relaxation, marked discoordination of pharyngeal pressurization, cricopharyngeal relaxation and cervical esophageal contraction, or a hypopharyngeal bolus pressure suggesting decreased compliance of the skeletal portion of the cervical esophagus. In many patients with cricopharyngeal dysfunction, including those with Zenker’s diverticulum, it has been difficult to consistently demonstrate a motility abnormality or discoordination of pharyngoesophageal events. The abnormality most apt to be present is a loss of compliance in the pharyngoesophageal segment manifested by an increased bolus pressure. Cook and colleagues have demonstrated an increased resistance to the movement of a bolus through what appears on manometry to be a completely relaxed cricopharyngeal sphincter. Using simultaneous manometry and videofluoroscopy, they showed that, in these patients, the cricopharyngeus is only partially relaxed; that is, the sphincter is relaxed enough to allow a drop of its pressure to esophageal baseline on manometry, but insufficiently relaxed to allow unimpaired passage of the bolus into the esophagus. This incomplete relaxation is due to a loss of compliance of the muscle in the pharyngoesophageal segment, and may be associated with a cricopharyngeal bar or Zenker’s diverticulum. This decreased compliance of the cricopharyngeal sphincter can be Figure 25-48. A. Schematic drawing of a pharyngeal pressure wave indicating the presence of the bolus pressure. B. Schematic drawing of the manometric recording typically seen during cricopharyngeal sphincter relaxation. recognized on esophageal manometry by a “shoulder” on the pharyngeal pressure wave, the amplitude of which correlates directly with the degree of outflow obstruction (Fig. 25-49). Increasing the diameter of this noncompliant segment reduces the resistance imposed on the passage of a bolus. Consequently, patients with low pharyngeal pressure (i.e., poor piston function of the pharynx), or patients with increased resistance of the pharyngocervical esophageal segment from loss of skeletal muscle compliance, are improved by a cricopharyngeal myotomy. This enlarges the pharyngoesophageal segment and reduces outflow resistance. Esophageal muscle biopsy specimens from patients with Zenker’s diverticulum have shown histologic evidence of the restrictive myopathy in the cricophayngeous muscle. These findings correlate well with the observation of a decreased compliance of the upper esophagus demonstrated by videoradiography and the findings on detailed manometric studies of the pharynx and cervical esophagus. They suggest that the diverticulum develops as a consequence of the outflow resistance to bolus transport through the noncompliant muscle of the pharyngoesophageal segment. The requirements for a successful pharyngoesophageal myotomy are (a) adequate oropharyngeal bolus transport; (b) the presence of an intact swallowing reflex; (c) reasonable coordination of pharyngeal pressurization with cricopharyngeal relaxation; and (d) a cricopharyngeal bar, Zenker’s diverticulum, or a narrowed pharyngoesophageal segment on videoesophagogram and/or the presence of excessive pharyngoesophageal shoulder pressure on motility study. Zenker’s Diverticulum. In the past, the most common recognized sign of cricopharyngeal dysfunction was the presence of a CHAPTER 25 ESOPHAGUS AND DIAPHRAGMATIC HERNIA A 40 200 30 150 Zenker’s 20 10 UES area mm2 Pharyngeal shoulder pressure mmHg 1054 Controls 0 5 10 15 Controls 100 Zenker’s 50 PART II 0 20 Swallow volume 5 10 15 20 SPECIFIC CONSIDERATIONS Zenker’s diverticulum, originally described by Ludlow in 1769. The eponym resulted from Zenker’s classic clinicopathologic descriptions of 34 cases published in 1878. Pharyngoesophageal diverticula have been reported to occur in 1 of 1000 routine barium examinations, and classically occur in elderly, white males. Zenker’s diverticula tend to enlarge progressively with time due to the decreased compliance of the skeletal portion of the cervical esophagus that occurs with aging. Presenting symptoms include dysphagia associated with the spontaneous regurgitation of undigested, bland material, often interrupting eating or drinking. On occasion, the dysphagia can be severe enough to cause debilitation and significant weight loss. Chronic aspiration and repetitive respiratory infection are common associated complaints. Once suspected, the diagnosis is established by a barium swallow. Endoscopy is usually difficult in the presence of a cricopharyngeal diverticulum, and potentially dangerous, owing to obstruction of the true esophageal lumen by the diverticulum and the attendant risk of diverticular perforation. Cricopharyngeal Myotomy. The low morbidity and mortality associated with cricopharyngeal and upper esophageal myotomy have encouraged a liberal approach toward its use for almost any problem in the oropharyngeal phase of swallowing. This attitude has resulted in an overall success rate in the relief of symptoms of only 64%. When patients are selected for surgery using radiographic or motility markers of disease, a much higher proportion will benefit. Two methods of cricopharyngoesophageal myotomy are in common use, one using traditional surgical approaches, and one using rigid laryngoscopy and a linear cutting stapler. mm Hg 40– Figure 25-49. Pharyngeal shoulder pressures and diameter of the pharyngoesophageal segment in controls and patients with Zenker’s diverticulum. UES = upper esophageal sphincter. (Data from Cook IJ, et al. Zenker’s diverticulum: evidence for a restrictive cricopharyngeal myopathy. Gastroenterology. 1989;96:A98.) Open Cricopharyngeal Myotomy, Diverticulopexy, and Diverticulectomy. The myotomy can be performed under local or general anesthesia through an incision along the anterior border of the left sternocleidomastoid muscle. The pharynx and cervical esophagus are exposed by retracting the sternocleidomastoid muscle and carotid sheath laterally and the thyroid, trachea, and larynx medially (Fig. 25-50). When a pharyngoesophageal diverticulum is present, localization of the pharyngoesophageal segment is easy. The diverticulum is carefully freed from the overlying areolar tissue to expose its neck, just below the inferior pharyngeal constrictor and above the cricopharyngeus muscle. It can be difficult to identify the cricopharyngeus muscle in the absence of a diverticulum. A benefit of local anesthesia is that the patient can swallow and demonstrate an area of persistent narrowing at the pharyngoesophageal junction. Furthermore, before closing the incision, gelatin can be fed to the patient to ascertain whether the symptoms have been relieved, and to inspect the opening of the previously narrowed pharyngoesophageal segment. Under general anesthesia, and in the absence of a diverticulum, the placement of a nasogastric tube to the level of the manometrically determined cricopharyngeal sphincter helps in localization of the structures. The myotomy is extended cephalad by dividing 1 to 2 cm of inferior constrictor muscle of the pharynx, and caudad by dividing the cricopharyngeal muscle and the cervical esophagus for a length of 4 to 5 cm. The cervical wound is closed only when all oozing of blood has ceased because a hematoma after this procedure is common and is often associated with temporary dysphagia while the hematoma absorbs. Oral alimentation is started the day after surgery. The patient is usually discharged on the first or second postoperative day. Hypopharynx 0 40 30 20 10 0 Cricopharyngeus Figure 25-50. Cross-section of the neck at the level of the thyroid isthmus that shows the surgical approach to the hypopharynx and cervical esophagus. (Reproduced with permission from Waters PF, DeMeester TR: Foregut motor disorders and their surgical management, Med Clin North Am. 1981 Nov;65(6):1235-1268.) 1055 Zenker’s diverticulum If a diverticulum is present and is large enough to persist after a myotomy, it may be sutured in the inverted position to the prevertebral fascia using a permanent suture (i.e., diverticulopexy) (Fig. 25-51). If the diverticulum is excessively large so that it would be redundant if suspended, or if its walls are thickened, a diverticulectomy should be performed. This is best performed under general anesthesia by placing a Maloney dilator (48F) in the esophagus, after controlling the neck of the diverticulum and after myotomy. A linear stapler is placed across the neck of the diverticulum, and the diverticulum is excised distal to the staple line. The security of this staple line and effectiveness of the myotomy may be tested before hospital discharge with a water-soluble contrast esophagogram. Postoperative complications include fistula formation, abscess, hematoma, recurrent nerve paralysis, difficulties in phonation, and Horner’s syndrome. The incidence of the first two can be reduced by performing a diverticulopexy rather than diverticulectomy. Endoscopic Cricopharyngotomy. Endoscopic stapled cricopharyngotomy and diverticulotomy recently has been described. This procedure is most effective for larger diverticula (>2 cm) and may be impossible to perform for the small diverticulum. The procedure uses a specialized “diverticuloscope” with two retractable valves passed into the hypopharynx. The lips of the diverticuloscope are positioned so that one lip lies in the esophageal lumen and the other in the diverticular lumen. The valves of the diverticuloscope are retracted appropriately so as to visualize the septum interposed between the diverticulum and the esophagus. An endoscopic linear stapler is introduced into the diverticuloscope and positioned against the common septum with the anvil in the diverticulum and the cartridge in the esophageal lumen. Firing of the stapler divides the common septum between the posterior esophageal and the diverticular wall over a length of 30 mm, placing three rows of staples on each side. More than one stapler application may be needed, depending on the size of the diverticulum (Fig. 25-52). The patient is allowed to resume liquid feeds immediately and is usually discharged the day after surgery. Complications are rare and may include perforation at the apex of the diverticulum and failure to relieve dysphagia resulting from incomplete myotomy. The former complication can usually be treated with antibiotics, but it may, rarely, require neck drainage. Recurrence of a Zenker’s diverticulum may occur with long follow-up and is more common after diverticulectomy without myotomy, presumably due to persistence of the underlying loss of compliance of the cervical esophagus when a myotomy is not performed. After endoscopic cricopharyngotomy Prevertebral fascia Figure 25-51. Posterior of the anatomy of the pharynx and cervical esophagus showing pharyngoesophageal myotomy and pexing of the diverticulum to the prevertebral fascia. lateral residual “pouches” may be seen on radiographs, but they are rarely responsible for residual or recurrent symptoms if the myotomy has been complete. Postoperative motility studies have shown that the peak pharyngeal pressure generated on swallowing is not affected, the resting cricopharyngeal pressure is reduced but not eliminated, and the cricopharyngeal sphincter length is shortened. Consequently, after myotomy, there is protection against esophagopharyngeal regurgitation. Motility Disorders of the Esophageal Body and Lower Esophageal Sphincter Disorders of the esophageal phase of swallowing result from abnormalities in the propulsive pump action of the esophageal body or the relaxation of the LES. These disorders result from either primary esophageal abnormalities, or from generalized neural, muscular, or collagen vascular disease (Table 25-8). The use of standard and high-resolution esophageal manometry techniques has allowed specific primary esophageal motility disorders to be identified out of a pool of nonspecific motility abnormalities. Primary esophageal motor disorders include achalasia, DES, nutcracker esophagus, and the hypertensive LES. The manometric characteristics of these disorders are shown in Table 25-9. The boundaries between the primary esophageal motor disorders are vague, and intermediate types exist, some of which may combine more than one type of motility pattern. These findings indicate that esophageal motility disorders should be looked at as a spectrum of abnormalities that reflects various stages of destruction of esophageal motor function. Achalasia. The best known and best understood primary motility disorder of the esophagus is achalasia, with an incidence of six Figure 25-52. The technique for transoral cricopharyngotomy and Zenker’s diverticulotomy. CHAPTER 25 ESOPHAGUS AND DIAPHRAGMATIC HERNIA Myotomy 1056 Table 25-8 Esophageal motility disorders Primary esophageal motility disorders Achalasia, “vigorous” achalasia Diffuse and segmental esophageal spasm Nutcracker esophagus Hypertensive lower esophageal sphincter Nonspecific esophageal motility disorders PART II SPECIFIC CONSIDERATIONS Secondary esophageal motility disorders Collagen vascular diseases: progressive systemic sclerosis, polymyositis and dermatomyositis, mixed connective tissue disease, systemic lupus erythematosus, etc. Chronic idiopathic intestinal pseudoobstruction Neuromuscular diseases Endocrine and metastatic disorders per 100,000 population per year. Although complete absence of in the esophageal body has been proposed as the 5 peristalsis major abnormality, present evidence indicates achalasia is a primary disorder of the LES. This is based on 24-hour outpatient esophageal motility monitoring, which shows that, even in advanced disease, up to 5% of contractions can be peristaltic. Table 25-9 Manometric characteristics of the primary esophageal motility disorders Achalasia Incomplete lower esophageal sphincter (LES) relaxation (<75% relaxation) Aperistalsis in the esophageal body Elevated LES pressure ≤26 mmHg Increased intraesophageal baseline pressures relative to gastric baseline Diffuse esophageal spasm (DES) Simultaneous (nonperistaltic contractions) (>20% of wet swallows) Repetitive and multipeaked contractions Spontaneous contractions Intermittent normal peristalsis Contractions may be of increased amplitude and duration Nutcracker esophagus Mean peristaltic amplitude (10 wet swallows) in distal esophagus ≥180 mmHg Increased mean duration of contractions (>7.0 s) Normal peristaltic sequence Hypertensive lower esophageal sphincter Elevated LES pressure (≥26 mmHg) Normal LES relaxation Normal peristalsis in the esophageal body Ineffective esophageal motility disorders Decreased or absent amplitude of esophageal peristalsis (<30 mmHg) Increased number of nontransmitted contractions Reproduced with permission from Zuidema GD, Orringer MB: Shackelford’s Surgery of the Alimentary Tract, 3rd ed. Vol 1. Philadelphia, PA: Elsevier/Saunders; 1991. Simultaneous esophageal waves develop as a result of the increased resistance to esophageal emptying caused by the nonrelaxing LES. This conclusion is supported by experimental studies in which a band placed loosely around the GEJ in experimental models did not change sphincter pressures but resulted in impaired relaxation of the LES and outflow resistance. This led to a markedly increased frequency of simultaneous waveforms and a decrease in contraction amplitude. The changes were associated with radiographic dilation of the esophagus and were reversible after removal of the band. Observations in patients with pseudoachalasia due to tumor infiltration, a tight stricture in the distal esophagus, or an antireflux procedure that is too tight also provide evidence that dysfunction of the esophageal body can be caused by the increased outflow obstruction of a nonrelaxing LES. The observation that esophageal peristalsis can return in patients with classic achalasia following dilation or myotomy provides further support that achalasia is a primary disease of the LES. The pathogenesis of achalasia is presumed to be a neurogenic degeneration, which is either idiopathic or due to infection. In experimental animals, the disease has been reproduced by destruction of the nucleus ambiguus and the dorsal motor nucleus of the vagus nerve. In patients with the disease, degenerative changes have been shown in the vagus nerve and in the ganglia in the myenteric plexus of the esophagus itself. This degeneration results in hypertension of the LES, a failure of the sphincter to relax on swallowing, elevation of intraluminal esophageal pressure, esophageal dilatation, and a subsequent loss of progressive peristalsis in the body of the esophagus. The esophageal dilatation results from the combination of a nonrelaxing sphincter, which causes a functional retention of ingested material in the esophagus, and elevation of intraluminal pressure from repetitive pharyngeal air swallowing (Fig. 25-53). With time, the functional disorder results in anatomic alterations seen on radiographic studies, such as a dilated esophagus with a tapering, “bird’s beak”-like narrowing of the distal end (Fig. 25-54). There is usually an airfluid level in the esophagus from the retained food and saliva, the height of which reflects the degree of resistance imposed by the nonrelaxing sphincter. As the disease progresses, the esophagus becomes massively dilated and tortuous. A subgroup of patients with otherwise typical features of classic achalasia has simultaneous contractions of their esophageal body that can be of high amplitude. This manometric pattern has been termed vigorous achalasia, and chest pain episodes are a common finding in these patients. Since the development of high resolution esophageal manometry technology, the term vigorous achalasia has been replaced with Chicago type 3 achalasia. Differentiation of type 3 achalasia from DES can be difficult. In both diseases, videoradiographic examination may show a corkscrew deformity of the esophagus and diverticulum formation. Diffuse and Segmental Esophageal Spasm. DES is characterized by substernal chest pain and/or dysphagia. DES differs from classic achalasia in that it is primarily a disease of the esophageal body, produces a lesser degree of dysphagia, causes more chest pain, and has less effect on the patient’s general condition. Nonetheless, it is impossible to differentiate achalasia from DES on the basis of symptoms alone. Esophagogram and esophageal manometry are required to distinguish these two entities. True symptomatic DES is a rare condition, occurring about five times less frequently than achalasia. The causation and neuromuscular pathophysiology of DES are unclear. The basic motor abnormality is rapid wave progression down the esophagus secondary to an abnormality in 1057 100 mmHg 10 mins 3 140 120 100 80 60 40 20 0 –20 10 secs 4 60 50 40 30 20 10 0 –10 –20 100 mmHg 6 45 35 * 25 15 9 –5 –15 –25 –35 A 3150 140 * 120 100 80 60 40 20 0 Meal 4150 140 * 120 100 80 60 40 20 0 5150 140 * 120 100 80 60 40 20 0 6145 125 * 105 100 85 65 45 5 –15 B Figure 25-53. Pressurization of esophagus: ambulatory motility tracing of a patient with achalasia. A. Before esophageal myotomy. B. After esophageal myotomy. The tracings have been compressed to exaggerate the motility spikes and baseline elevations. Note the rise in esophageal baseline pressure during a meal represented by the rise off the baseline to the left of panel A. No such rise occurs postmyotomy (B). the latency gradient. Hypertrophy of the muscular layer of the esophageal wall and degeneration of the esophageal branches of the vagus nerve have been observed in this disease, although these are not constant findings. Manometric abnormalities in DES may be present over the total length of the esophageal body but usually are confined to the distal two-thirds. In segmental esophageal spasm, the manometric abnormalities are confined to a short segment of the esophagus. The classic manometric findings in these patients are characterized by the frequent occurrence of simultaneous waveforms and multipeaked esophageal contractions, which may be of abnormally high amplitude or long duration. Key to the diagnosis of DES is that there remain some peristaltic waveforms in excess of those seen in achalasia. A criterion of 30% or more peristaltic waveforms out of 10 wet swallows has been used to differentiate DES from vigorous achalasia. However, this figure is arbitrary and often debated. The LES in patients with DES usually shows a normal resting pressure and relaxation on swallowing. A hypertensive sphincter with poor relaxation may also be present. In patients with advanced disease, the radiographic appearance of tertiary contractions appears helical and has been termed corkscrew Figure 25-54. Barium esophagogram showing a markedly dilated esophagus and characteristic “bird’s beak” in achalasia. (Reproduced with permission from Waters PF, DeMeester TR: Foregut motor disorders and their surgical management, Med Clin North Am. 1981 Nov;65(6):1235-1268.) esophagus or pseudodiverticulosis (Fig. 25-55). Patients with segmental or diffuse esophageal spasm can compartmentalize the esophagus and develop an epiphrenic or midesophageal diverticulum between two areas of high pressure occurring simultaneously (Fig. 25-56). Nutcracker Esophagus. The disorder, termed nutcracker or supersqueezeresophagus, was recognized in the late 1970s. Other terms used to describe this entity are hypertensive peristalsis or high-amplitude peristaltic contractions. It is the most common of the primary esophageal motility disorders. By definition the so-called nutcracker esophagus is a manometric abnormality in patients who are characterized by peristaltic esophageal contractions with peak amplitudes greater than two SDs above the normal values in individual laboratories. Contraction amplitudes in these patients can easily be above 400 mmHg. At the lower end of peak pressure, it is unclear whether nutcracker esophagus causes any symptoms. In fact, chest pain symptoms in nutcracker esophagus patients may be related to GERD rather than intraluminal hypertension. Treatment in these patients should be aimed at the treatment of GERD. At the high end (peak pressures >300 mmHg) chest pain may be the result of the nutcracker physiology, as treatment directed at reducing intraluminal pressure is more effective than when used for those with lower peak pressures. Hypertensive Lower Esophageal Sphincter. Hypertensive lower esophageal sphincter (LES) in patients with chest pain or dysphagia was first described as a separate entity by Code and associates. This disorder is characterized by an elevated basal pressure of the LES with normal relaxation and CHAPTER 25 ESOPHAGUS AND DIAPHRAGMATIC HERNIA 5 60 50 40 30 20 10 0 –10 –20 1058 PART II normal propulsion in the esophageal body. About one-half of these patients, however, have associated motility disorders of the esophageal body, particularly hypertensive peristalsis and simultaneous waveforms. In the remainder, the disorder exists as an isolated abnormality. Dysphagia in these patients may be caused by a lack of compliance of the sphincter, even in its relaxed state. Myotomy of the LES may be indicated in patients not responding to medical therapy or dilation. When the symptom contribution of the hypertensive sphincter is in doubt, it is possible to inject the LES with botulinum toxin, endoscopically. If symptoms are relieved (temporarily) with this technique, then it is likely that myotomy will provide more permanent benefit. SPECIFIC CONSIDERATIONS Secondary Esophageal Motility Disorders. Connective tissue disease, particularly scleroderma and the CREST syndrome, exhibits severe esophageal motility disorders. Additionally, patients treated as infants for esophageal atresia will often develop secondary motility disorders manifest later in life. Symptoms of these disorders are heartburn and dysphagia. The latter may be a result of a peptic stricture rather than the esophageal dysmotility. An esophageal motility study will usually show severely reduced or absent peristalsis with severely reduced or absent LES pressure. The role of antireflux surgery under these conditions is controversial but, if performed, should be limited to partial fundoplication, as full (Nissen) fundoplication may result in severe dysphagia. Figure 25-55. Barium esophagogram of patient with diffuse spasm showing the corkscrew deformity. Nonspecific Esophageal Motor Disorders and Ineffective Esophageal Motility. Many patients complaining of dys- Figure 25-56. Barium esophagogram showing a high epiphrenic diverticulum in a patient with diffuse esophageal spasm. (Reproduced with permission from Castell DO: The Esophagus. Boston, MA: Little, Brown; 1992.) Diverticula of the Esophageal Body. Diverticula of the esophagus may be characterized by their location in the esophagus (proximal, mid-, or distal esophagus), or by the nature of phagia or chest pain of noncardiac origin demonstrate a variety of wave patterns and contraction amplitudes on esophageal manometry that are clearly out of the normal range, but do not meet the criteria of a primary esophageal motility disorder. Esophageal motility in these patients frequently shows an increased number of multipeaked or repetitive contractions, contractions of prolonged duration, nontransmitted contractions, an interruption of a peristaltic wave at various levels of the esophagus, or contractions of low amplitude. These motility abnormalities have been termed nonspecific esophageal motility disorders. Their significance in the causation of chest pain or dysphagia is still unclear. Surgery plays no role in the treatment of these disorders unless there is an associated diverticulum. A clear distinction between primary esophageal motility disorders and nonspecific esophageal motility disorders is often not possible. Patients diagnosed as having nonspecific esophageal motility abnormalities on repeated studies will occasionally show abnormalities consistent with nutcracker esophagus. Similarly, progression from a nonspecific esophageal motility disorder to classic DES has been demonstrated. Therefore, the finding of a nonspecific esophageal motility disorder may represent only a manometric marker of an intermittent, more severe esophageal motor abnormality. Combined ambulatory 24-hour esophageal pH and motility monitoring has shown that an increased esophageal exposure to gastric juice is common in patients diagnosed as having a nonspecific esophageal motility disorder. In some situations, the motor abnormalities may be induced by the irritation of refluxed gastric juice; in other situations, it may be a primary event unrelated to the presence of reflux. High-amplitude peristalsis (nutcracker esophagus) and low-amplitude peristalsis (ineffective esophageal motility) are frequently associated with GERD. 1059 Figure 25-57. Barium esophagogram showing a midesophageal diverticulum. Despite the anatomic distortion, the patient was asymptomatic. (Reproduced with permission from Waters PF, DeMeester TR: Foregut motor disorders and their surgical management, Med Clin North Am. 1981 Nov;65(6):1235-1268.) sarcoid, may create traction esophageal diverticula after successful treatment. Rarely, when no underlying inflammatory pathology is identified, a motility disorder may be identified. Most midesophageal diverticula are asymptomatic and incidentally discovered during investigation for nonesophageal complaints. In such patients, the radiologic abnormality may Inflamed nodes Traction diverticulum Figure 25-58. Illustration of the pathophysiology of midesophageal diverticulum showing traction on the esophageal wall from adhesions to inflamed subcarinal lymph nodes. CHAPTER 25 ESOPHAGUS AND DIAPHRAGMATIC HERNIA concomitant pathology. Diverticula associated with motor disorders are termed pulsion diverticula and those associated with inflammatory conditions are termed traction diverticula. Pulsion diverticula occur most commonly with nonspecific motility disorders, but they can occur with all of the primary motility disorders. In the latter situation, the motility disorder is usually diagnosed before the development of the diverticulum. When associated with achalasia, the development of a diverticulum may temporarily alleviate the symptom of dysphagia by becoming a receptacle for ingested food and substitute the symptom of dysphagia for postprandial pain and regurgitation of undigested food. If a motility abnormality of the esophageal body or LES cannot be identified, a traction or congenital cause for the diverticulum should be considered. Because development in radiology preceded development in motility monitoring, diverticula of the esophagus were considered historically to be a primary abnormality, the cause, rather than the consequence, of motility disorders. Consequently, earlier texts focused on them as specific entities based upon their location. Epiphrenic diverticula arise from the terminal third of the thoracic esophagus and are usually found adjacent to the diaphragm. They have been associated with distal esophageal muscular hypertrophy, esophageal motility abnormalities, and increased luminal pressure. They are “pulsion” diverticula, and they are associated with diffuse spasm, achalasia, or nonspecific motor abnormalities in the body of the esophagus. Whether the diverticulum should be surgically resected or suspended depends on its size and proximity to the vertebral body. When diverticula are associated with esophageal motility disorders, esophageal myotomy from the proximal extent of the diverticulum to the stomach should be combined with diverticulectomy. If diverticulectomy alone is performed, one can expect a high incidence of suture line rupture due to the same intraluminal pressure that initially gave rise to the diverticulum. If the diverticulum is suspended to the prevertebral fascia of the thoracic vertebra, a myotomy is begun at the neck of the diverticulum and extended across the LES. If the diverticulum is excised by dividing the neck, the muscle is closed over the excision site, and a myotomy is performed on the opposite esophageal wall, starting just above the level of the diverticulum or at the proximal extent of the spastic segment of the esophagus if high resolution motility is used. If complete, the myotomy will cross the LES, reducing distal esophageal peak pressure, and it will increase the likelihood that dysphagia will be replaced with GERD symptoms. Increasingly, partial fundoplication (anterior or posterior) is performed after LES myotomy to decrease the frequency of disabling GERD developing after myotomy and diverticulectomy. When a large diverticulum is associated with a hiatal hernia, then hiatal hernia repair is added. All these procedures may be performed with traditional or minimally invasive techniques. Midesophageal or traction diverticula were first described in the 19th century (Fig. 25-57). At that time, they were frequently noted in patients who had mediastinal LN involvement with tuberculosis. It was theorized that adhesions formed between the inflamed mediastinal nodes and the esophagus. By contraction, the adhesions exerted traction on the esophageal wall and led to a localized diverticulum (Fig. 25-58). This theory was based on the findings of early dissections, where adhesions between diverticula and LNs were commonly found. Other conditions associated with mediastinal lymphadenopathy, such as pulmonary fungal infections (e.g., aspergillosis), lymphoma, or 1060 PART II be ignored. Patients with symptoms of dysphagia, regurgitation, chest pain, or aspiration, in whom a diverticulum is discovered, should be thoroughly investigated for an esophageal motor abnormality. Occasionally, a patient will present with a bronchoesophageal fistula manifested by a chronic cough on ingestion of meals. The diverticulum in such patients is most likely to have an inflammatory etiology. The indication for surgical intervention is dictated by the degree of symptomatic disability. Usually, midesophageal diverticula can be suspended due to their proximity to the spine. If a motor abnormality is documented, a myotomy should be performed as described for an epiphrenic diverticulum. OPERATIONS FOR ESOPHAGEAL MOTOR DISORDERS AND DIVERTICULA SPECIFIC CONSIDERATIONS Long Esophageal Myotomy for Motor Disorders of the Esophageal Body A long esophageal myotomy is indicated for dysphagia caused by any motor disorder characterized by segmental or generalized simultaneous waveforms in a patient whose symptoms are not relieved by medical therapy. Such disorders include diffuse and segmental esophageal spasm, vigorous or type 3 achalasia, and nonspecific motility disorders associated with a mid- or epiphrenic esophageal diverticulum. However, the decision to operate must be made by a balanced evaluation of the patient’s symptoms, diet, lifestyle adjustments, and nutritional status, with the most important factor being the possibility of improving the patient’s swallowing disability. The symptom of chest pain alone is not an indication for a surgical procedure. The identification of patients with symptoms of dysphagia and chest pain who might benefit from a surgical myotomy is difficult. Ambulatory motility studies have shown that when the prevalence of “effective contractions” (i.e., peristaltic waveforms consisting of contractions with an amplitude above 30 mmHg) drops below 50% during meals, the patient is likely to experience dysphagia (Fig. 25-59). This would suggest that relief from the symptom can be expected with an improvement of esophageal contraction amplitude or amelioration of nonperistaltic waveforms. Prokinetic agents may increase esophageal contraction amplitude, but they do not alter the prevalence of simultaneous waveforms. Patients in whom the efficacy of esophageal propulsion is severely compromised because of a 100% 80% 60% 40% 20% 0% Normal volunteers Pat, no dysphagia Pat, dysphagia Figure 25-59. Prevalence of effective contractions (i.e., peristaltic contractions with an amplitude >30 mmHg) during meal periods in individual normal volunteers, patients (Pat) without dysphagia, and patients with nonobstructive dysphagia. 100% % Symptomatic 80% 60% 40% 10 cm % Retention 20% Eso. diameter 0% N Pre Rx 0–24 25–48 49–72 73–120 mo mo mo mo 17 17 16 14 12 5 cm 0 cm Figure 25-60. Esophageal (Eso.) diameter, dysphagia, and esophageal retention in patients with achalasia treated with myotomy and Nissen fundoplication, 10 years after treatment (Rx). (Data from Topart P, Deschamps C, Taillefer R, et al: Long-term effect of total fundoplication on the myotomized esophagus, Ann Thorac Surg. 1992 Dec;54(6):1046-1051.) high prevalence of simultaneous waveforms usually receive little benefit from medical therapy. In these patients, a surgical myotomy of the esophageal body can improve the patients’ dysphagia, provided the loss of contraction amplitude in the remaining peristaltic waveforms, caused by the myotomy, has less effect on swallowing function than the presence of the excessive simultaneous contractions. This situation is reached when the prevalence of effective waveforms during meals drops below 30% (i.e., 70% of esophageal waveforms are ineffective). In patients selected for surgery, preoperative highresolution manometry is essential to determine the proximal extent of the esophageal myotomy. Most surgeons extend the myotomy distally across the LES to reduce outflow resistance. Consequently, some form of antireflux protection is needed to avoid gastroesophageal reflux if there has been extensive dissection of the cardia. In this situation, most authors prefer a partial, rather than a full, fundoplication, in order not to add back-resistance that will further interfere with the ability of the myotomized esophagus to empty (Fig. 25-60). If the symptoms of reflux are present preoperatively, 24-hour pH monitoring is required to confirm its presence. The procedure may be performed either open or via thoracoscopy. The open technique is performed through a left thoracotomy in the sixth intercostal space (Fig. 25-61). An incision is made in the posterior mediastinal pleura over the esophagus, and the left lateral wall of the esophagus is exposed. The esophagus is not circumferentially dissected unless necessary. A 2-cm incision is made into the abdomen through the parietal peritoneum at the midportion of the left crus. A tongue of gastric fundus is pulled into the chest. This exposes the GEJ and its associated fat pad. The latter is excised to give a clear view of the junction. A myotomy is performed through all muscle layers, extending distally over the stomach 1 to 2 cm below the GEJ, and proximally on the esophagus over the distance of the manometric abnormality. The muscle layer is dissected from the mucosa laterally for a distance of 1 cm. Care is taken to divide all minute muscle bands, particularly in the area of the GEJ. The gastric fundic tongue is sutured to the margins of the myotomy over a distance of 3 to 4 cm and replaced into the abdomen. This maintains separation of the muscle and acts as a partial fundoplication to prevent reflux. 1061 CHAPTER 25 ESOPHAGUS AND DIAPHRAGMATIC HERNIA Figure 25-61. Technique of long myotomy: A. Exposure of the lower esophagus through the left sixth intercostal space and incision of the mediastinal pleura in preparation for surgical myotomy. B. Location of a 2-cm incision made through the phrenoesophageal membrane into the abdomen along the midlateral border of the left crus. C. Retraction of tongue of gastric fundus into the chest through the previously made incision. D. Removal of the gastroesophageal fat pad to expose the gastroesophageal junction. E. A myotomy down to the mucosa is started on the esophageal body. F. Completed myotomy extending over the stomach for 1 cm. G. Reconstruction of the cardia after a myotomy, illustrating the position of the sutures used to stitch the gastric fundic flap to the margins of the myotomy. H. Reconstruction of the cardia after a myotomy, illustrating the intra-abdominal position of the gastric tongue covering the distal 4 cm of the myotomy. 1062 PART II SPECIFIC CONSIDERATIONS Figure 25-61. (Continued ) If an epiphrenic diverticulum is present, it is excised by dividing the neck with a stapler sized for the thickness of the diverticulum (2.0- to 4.8-mm staple leg length) followed by a closure of the muscle over the staple line, when possible. The myotomy is then performed on the opposite esophageal wall. If a midesophageal diverticulum is present, the myotomy is made so that it includes the muscle around the neck, and the diverticulum is suspended by attaching it to the paravertebral fascia of the thoracic vertebra above the level of the diverticular neck. Before performing any operation for an esophageal diverticulum, it is wise to endoscope the patient to wash all food and other debris from the diverticulum. The results of myotomy for motor disorders of the esophageal body have improved in parallel with the improved preoperative diagnosis afforded by manometry. Previous published series report between 40% and 92% improvement of symptoms, but interpretation is difficult due to the small number of patients involved and the varying criteria for diagnosis of the primary motor abnormality. When myotomy is accurately done, 93% of the patients have effective palliation of dysphagia after a mean followup of 5 years, and 89% would have the procedure again, if it was necessary. Most patients gain or maintain rather than lose weight after the operation. Postoperative motility studies show that the myotomy reduces the amplitude of esophageal contractions to near zero and eliminates simultaneous peristaltic waves. If the benefit of obliterating the simultaneous waves exceeds the adverse effect on bolus propulsion caused by the loss of peristaltic waveforms, the patient’s dysphagia is likely to be improved by the procedure. If not, the patient is likely to continue to complain of dysphagia and to have little improvement as a result of the operation. The thoracoscopic technique may be performed through the left or right chest. There has been little experience gained with doing adequate operations (as described previously with the open exposure) through left thoracoscopy, so most surgeons will combine a right thoracoscopic long myotomy with an abdominal approach for Heller myotomy and partial fundoplication. These two procedures may be done at the same setting, by double positioning the patient, or they may be done at two operations. If this is the case, it is best to do the abdominal component first, as the esophageal outflow obstruction is the source of most of the symptoms. Performing abdominal myotomy (and diverticulectomy, if present) may be all that is required. A new procedure, peroral endoscopic myotomy (POEM) allows a long myotomy to be performed from the lumen of the esophagus with an endoscope. This procedure is attractive for, at a minimum, those with type 3 achalasia (vigorous achalasia), where it is necessary to divide esophagogastric circular muscle on both sides of the diaphragm to the extent that might not be possible with laparoscopy or thoracoscopy alone. The POEM procedure is started by opening the esophageal mucosa several centimeters above the spastic segment with a needle–knife electrosurgery device passed through an endoscope. A long submucosal plane is developed with the endoscope, down to and below the LES. The circular muscle of the LES and the esophagus is divided with endoscopic electrosurgery all the way back until normal (nonspastic) esophagus is reached. The submucosal entry site in the esophagus is then closed with endoscopic clips. While the results of POEM are still accumulating, the procedure is attractive because it is extremely minimally invasive and can be done on an outpatient basis. Epiphrenic diverticula cannot be treated with POEM and are most frequently addressed with laparoscopic access, in combination with a laparoscopic division of the LES (Heller myotomy) (Fig. 25-62). If the diverticulum can be completely mobilized through the hiatus, it may be safely excised from below. The neck of the diverticulum is transected with a GIA stapler after passage of a 48F dilator. Not infrequently, the diverticulum is sufficiently large that access to the neck of the diverticulum across the hiatus is quite difficult. Additionally, the inflammatory reaction to the diverticulum may further make the transhiatal dissection difficult. Under these circumstances, it is safer to perform the diverticulectomy through a right thoracoscopic approach either at the time of the initial procedure or at a later date, depending upon the frailty of the patient. Following diverticulectomy, it is critical that the esophageal staple line be treated with a great deal of care. Closure of the muscle over the staple line is preferable. Additionally, the patient is kept NPO or on clear liquids for 5 to 7 days, and a contrast study is obtained before advancing to a full liquid or “mushy food” diet. Solid foods are withheld for 2 weeks to decrease the likelihood of staple line leak. Buttressing or sealing the staple line with fibrin glue is also an attractive option. 1063 CHAPTER 25 ESOPHAGUS AND DIAPHRAGMATIC HERNIA Figure 25-62. A. Epiphrenic diverticula are situated above the lower esophageal sphincter on right side of esophagus. B. Stapler amputates neck of diverticulum. C. Muscle reapproximated over staple line, and Heller myotomy is performed. Myotomy of the Lower Esophageal Sphincter (Heller Myotomy) Second only to reflux disease, achalasia is the most common functional disorder of the esophagus to require surgical intervention. The goal of treatment is to relieve the functional outflow obstruction secondary to the loss of relaxation and compliance of the LES. This requires disrupting the LES muscle. When performed adequately (i.e., reducing sphincter pressure to <10 mmHg), and done early in the course of disease, LES myotomy results in symptomatic improvement with the occasional return of esophageal peristalsis. Reduction in LES resistance can be accomplished intraluminally by hydrostatic balloon dilation, which ruptures the sphincter muscle, by botulinum toxin injection, or by a surgical myotomy that cuts the sphincter. The difference between these three methods appears to be the greater likelihood of reducing sphincter pressure to <10 mmHg by surgical myotomy compared with hydrostatic balloon dilation. However, patients whose sphincter pressure has been reduced by hydrostatic balloon dilation to <10 mmHg have an outcome similar to those after surgical myotomy (Fig. 25-63). Botulinum toxin injection may achieve similar results, but it has a longer duration of action that may be measured in weeks or months, rather than years. Botulinum toxin injection may best be used as a diagnostic tool, when it is not clear whether a hypertensive LES is the primary cause of dysphagia. Responsiveness to botulinum toxin injection may predict a good response to Heller myotomy. The therapeutic decisions regarding the treatment of patients with achalasia center on four issues. The first issue is the question of whether newly diagnosed patients should be treated with pneumatic dilation or a surgical myotomy. Longterm follow-up studies have shown that pneumatic dilation 1064 1 % in remission 0.8 0.6 LES < 10 mmHg 0.53 LES > 10 mmHg 0.23 0.4 0.2 PART II 0 0 12 24 26 48 Months 60 72 84 96 SPECIFIC CONSIDERATIONS Figure 25-63. Prevalence of clinical remission in 122 patients stratified according to postdilatation lower esophageal sphincter (LES) pressures greater than or <10 mmHg. (Reproduced with permission from Ponce J, Garrigues V, Pertejo V, et al: Individual prediction of response to pneumatic dilation in patients with achalasia, Dig Dis Sci. 1996 Nov;41(11):2135-2141.) achieves adequate relief of dysphagia and pharyngeal regurgitation in 50% to 60% of patients (Fig. 25-64). Close follow-up is required, and if dilation fails, myotomy is indicated. For those patients who have a dilated and tortuous esophagus or an associated hiatal hernia, balloon dilation is dangerous and surgery is the better option. The outcome of the one controlled randomized study (38 patients) comparing the two modes of therapy suggests that surgical myotomy as a primary treatment gives better long-term results. Several randomized trials comparing laparoscopic cardiomyotomy with balloon dilation or botulinum toxin injection have favored the surgical approach as well. 100 90 80 70 60 % 50 40 30 20 10 0 Myotomy n = 81 Myotomy n = 65 Myotomy + antireflux n = 22 Pneumatic dilatation n = 122 Pneumatic dilatation n = 54 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 Years Figure 25-64. Summary of long-term studies reporting the proportion of patients with complete relief or minimal dysphagia (Stage 0–1) stratified according to type of treatment. (Data from: Ellis FH, Jr. Oesophagomyotomy for achalasia: a 22-year experience. Br J Surg. 1993;80:882; Goulbourne IA, Walbaum PR. Long-term results of Heller’s operation for achalasia. J Royal Coll Surg. 1985;30:101; Malthaner RA, Todd TR, Miller L, et al. Longterm results in surgically managed esophageal achalasia. Ann Thorac Surg. 1994;58:1343; Ponce J, Garrigues V, Pertejo V, et al. Individual prediction of response to pneumatic dilation in patients with achalasia. Dig Dis Sci. 1996;41:2135; Eckardt V, Aignherr C, Bernhard G. Predictors of outcome in patients with achalasia treated by pneumatic dilation. Gastroenterology. 1992;103:1732.) Although it has been reported that a myotomy after previous balloon dilation is more difficult, this has not been the experience of these authors unless the cardia has been ruptured in a sawtooth manner. In this situation, operative intervention, either immediately or after healing has occurred, can be difficult. Similarly, myotomy after botulinum toxin injection has reported to be more difficult, but this is largely a function of the submucosal inflammatory response, which may be a bit unpredictable, and is most intense in the first 6 to 12 weeks after injection. It is important to wait at least 3 months after botulinum toxin injection to perform cardiomyotomy to minimize the risk of encountering dense inflammation. The second issue is the question of whether a surgical myotomy should be performed through the abdomen or the chest. Myotomy of the LES can be accomplished via either an abdominal or thoracic approach. In the absence of a previous upper abdominal surgery, most surgeons prefer the abdominal approach to LES myotomy as laparoscopy results in less pain and a shorter length of stay than thoracoscopy. In addition, it is a bit easier to ensure a long gastric myotomy when the approach is transabdominal. The third issue—and one that has been long debated—is the question of whether an antireflux procedure should be added to a surgical myotomy. Excellent results have been reported following meticulously performed myotomy without an antireflux component. Retrospective studies, with long-term follow-up of large cohorts of patients undergoing Heller myotomy demonstrated that, after 10 years, more than 50% of patients had reflux symptoms without a fundoplication. In a recent randomized clinical trial, 7% of patients undergoing Dor fundoplication following LES myotomy had abnormal 24-hour pH probes, and 42% of patients with a myotomy only had abnormal reflux profiles. If an antireflux procedure is used as an adjunct to esophageal myotomy, a complete 360° fundoplication should be avoided. Rather, a 270° Belsey fundoplication, a Toupet posterior 180° fundoplication, or a Dor anterior 180° fundoplication should be used to avoid the long-term esophageal dysfunction secondary to the outflow obstruction afforded by the fundoplication itself. The fourth issue centers on whether or not a cure of this disease is achievable. Long-term follow-up studies after surgical myotomy have shown that late deterioration in results occurs after this procedure, regardless of whether an antireflux procedure is done, and also after balloon dilation, even when the sphincter pressure is reduced to below 10 mmHg. It may be that, even though a myotomy or balloon rupture of the LES muscle reduces the outflow obstruction at the cardia, the underlying motor disorder in the body of the esophagus persists and deteriorates further with the passage of time, leading to increased impairment of esophageal emptying. The earlier an effective reduction in outflow resistance can be accomplished, the better the outcome will be, and the more likely some esophageal body function can be restored. In performing a surgical myotomy of the LES, there are four important principles: (a) complete division of all circular and collar-sling muscle fibers, (b) adequate distal myotomy to reduce outflow resistance, (c) “undermining” of the muscularis to allow wide separation of the esophageal muscle, and (d) prevention of postoperative reflux. In the past, the drawback of a surgical myotomy was the need for an open procedure, which often deterred patients from choosing the best treatment option for achalasia. With the advent of minimally invasive surgical techniques two decades ago, laparoscopic cardiomyotomy (Heller myotomy) has become the treatment of choice for most patients with achalasia. Open Esophageal Myotomy Laparoscopic Cardiomyotomy More commonly known as a laparoscopic Heller myotomy, after Ernst Heller, a German surgeon who described a “double myotomy” in 1913, the laparoscopic approach is similar to the Nissen fundoplication in terms of the trocar placement and exposure and dissection of the esophageal hiatus (Fig. 25-65). The procedure begins by division of the short gastric vessels Per Oral Endoscopic Myotomy (POEM) The POEM procedure was developed in Japan. It is the ultimate minimally invasive myotomy as it requires no incisions through the skin. With the POEM procedure, a very effective myotomy is performed entirely from the lumen of the esophagus. The POEM procedure is started by opening the esophageal mucosa 10 cm above the lower esophageal sphincter with a needle–knife electrosurgery device passed through an endoscope. A long submucosal plane is developed with the endoscope, down to and below the LES. The circular muscle of the LES, above and below the gastroesophageal junction, is divided with endoscopic electrosurgery. The submucosal entry site in the esophagus is then closed with endoscopic clips. While the results of POEM are still accumulating, the procedure is attractive because it is extremely minimally invasive, and can be done on an outpatient basis. The major downside of POEM is that an effective antireflux valve cannot be created, exposing the patient to a 40% to 50% risk of GERD post procedure. Outcome Assessment of the Therapy for Achalasia Critical analysis of the results of therapy for motor disorders of the esophagus requires objective measurement. The use of symptoms alone as an endpoint to evaluate therapy for achalasia may be misleading. The propensity for patients to unconsciously modify their diet to avoid difficulty swallowing is underestimated, making an assessment of results based on symptoms unreliable. Insufficient reduction in outflow resistance may allow progressive esophageal dilation to develop slowly, giving the impression of improvement because the volume of food able to be ingested with comfort increases. A variety of objective measurements may be used to assess success, including LES pressure, esophageal baseline pressure, and scintigraphic assessment of esophageal emptying time. Esophageal baseline pressure is usually negative compared to gastric pressure. Given that the goal of therapy is to eliminate the outflow resistance of a nonrelaxing sphincter, measurements of improvements in esophageal baseline pressure and scintigraphic transit time may be better indicators of success, but these are rarely reported. 1065 CHAPTER 25 ESOPHAGUS AND DIAPHRAGMATIC HERNIA Open techniques of distal esophageal myotomy are rarely used outside reoperations. In fact, primary procedures can almost always be successfully completed via laparoscopy. A modified Heller myotomy can be performed through a left thoracotomy incision in the sixth intercostal space along the upper border of the seventh rib. The esophagus and a tongue of gastric fundus are exposed as described for a long myotomy. A myotomy through all muscle layers is performed, extending distally over the stomach to 1 to 2 cm below the junction, and proximally on the esophagus for 4 to 5 cm. The cardia is reconstructed by suturing the tongue of gastric fundus to the margins of the myotomy to prevent rehealing of the myotomy site and to provide reflux protection in the area of the divided sphincter. If an extensive dissection of the cardia has been done, a more formal Belsey repair is performed. The tongue of gastric fundus is allowed to retract into the abdomen. Traditionally, nasogastric drainage is maintained for 6 days to prevent distention of the stomach during healing. An oral diet is resumed on the seventh day, after a barium swallow study shows unobstructed passage of the bolus into the stomach without extravasation. In a randomized, long-term follow-up by Csendes and colleagues of 81 patients treated for achalasia, either by forceful dilation or by surgical myotomy, myotomy was associated with a significant increase in the diameter at the GEJ and a decrease in the diameter at the middle third of the esophagus on follow-up radiographic studies. There was a greater reduction in sphincter pressure and improvement in the amplitude of esophageal contractions after myotomy. After dilation, 13% of patients regained some peristalsis, compared with 28% after surgery. These findings were shown to persist over a 5-year follow-up period, at which time 95% of those treated with surgical myotomy were doing well. Of those who were treated with dilation, only 54% were doing well, while 16% required redilation, and 22% eventually required surgical myotomy to obtain relief. If simultaneous esophageal contractions are associated with the sphincter abnormality, the so-called vigorous achalasia, then the myotomy should extend over the distance of the abnormal motility as mapped by the preoperative motility study. Failure to do this will result in continuing dysphagia and a dissatisfied patient. The best objective evaluation of improvement in the patient following either balloon dilation or myotomy is a scintigraphic measurement of esophageal emptying time. A good therapeutic response improves esophageal emptying toward normal. However, some degree of dysphagia may persist despite improved esophageal emptying, due to disturbances in esophageal body function. When an antireflux procedure is added to the myotomy, it should be a partial fundoplication. A 360° fundoplication is associated with progressive retention of swallowed food, regurgitation, and aspiration to a degree that exceeds the patient’s preoperative symptoms. in preparation for fundoplication. Exposure of the GEJ via removal of the gastroesophageal fat pad follows. The anterior vagus nerve is swept right laterally along with the fat pad. Once completed, the GEJ and distal 4 to 5 cm of esophagus should be bared of any overlying tissue, and generally follows dissection of the GEJ. A distal esophageal myotomy is performed. It is generally easiest to begin the myotomy 1 to 2 cm above the GEJ, in an area above that of previous botulinum toxin injections or balloon dilation. Either scissors or a hook-type electrocautery can be used to initiate the incision in the longitudinal and circular muscle. Distally, the myotomy is carried across the GEJ and onto the proximal stomach for approximately 2 to 3 cm. After completion, the muscle edges are separated bluntly from the esophageal mucosa for approximately 50% of the esophageal circumference. An antireflux procedure follows completion of the myotomy. Either an anterior hemifundoplication augmenting the angle of His (Dor) or posterior partial fundoplication (Toupet) can be performed. The Dor type fundoplication is slightly easier to perform, and it does not require disruption of the normal posterior gastroesophageal attachments (a theoretical advantage in preventing postoperative reflux). 1066 PART II SPECIFIC CONSIDERATIONS Eckardt and associates investigated whether the outcome of pneumatic dilation in patients with achalasia could be predicted on the basis of objective measurements. Postdilation LES pressure was the most valuable measurement for predicting long-term clinical response. A postdilatation sphincter pressure <10 mmHg predicted a good response. Approximately 50% of the patients studied had postdilatation sphincter pressures between 10 and 20 mmHg, with a 2-year remission rate of 71%. More important, 16 of 46 patients were left with a postdilatation sphincter pressure of >20 mmHg and had an unacceptable outcome. Overall, only 30% of patients dilated remained in symptomatic remission at 5 years. Bonavina and colleagues reported good to excellent results with transabdominal myotomy and Dor fundoplication in 94% of patients after a mean follow-up of 5.4 years. No operative mortality occurred in either of these series, attesting to the safety of the procedure. Malthaner and Pearson reported the long-term clinical results in 35 patients with achalasia, having a minimum follow-up of 10 years (Table 25-10). Twenty-two of these patients underwent primary esophageal myotomy and Belsey hemifundoplication at the Toronto General Hospital. Excellent to good results were noted in 95% of patients at 1 year, declining to 68%, 69%, and 67% at 10, 15, and 20 years, respectively. Two patients underwent early reoperation for an incomplete myotomy, and three underwent an esophagectomy for progressive disease. They concluded that there was a deterioration of the initially good results after surgical myotomy and hiatal repair for achalasia, which is due to late complications of gastroesophageal reflux. Ellis reported his lifetime experience with transthoracic short esophageal myotomy without an antireflux procedure. One hundred seventy-nine patients were analyzed at a mean followup of 9 years, ranging from 6 months to 20 years. Overall, 89% of patients were improved at the 9-year mark. He also observed that the level of improvement deteriorated with time, with excellent results (patients continuing to be symptom free) decreasing from 54% at 10 years to 32% at 20 years. He concluded that a short transthoracic myotomy without an antireflux procedure provides excellent long-term relief of dysphagia, and, contrary to Malthaner and Pearson’s experience, does not result in complications of gastroesophageal reflux. Both studies document nearly identical results 10 to 15 years following the procedure, and both report deterioration over time, probably due to progression of the underlying disease. The addition of an antireflux procedure if the operation is performed transthoracically has no significant effect on the outcome. Figure 25-65. A. Longitudinal muscle is divided. B. Mechanical disruption of lower esophageal sphincter muscle fibers. C. Myotomy must be carried across gastroesophageal junction. D. Gastric extension should equal 2 to 3 cm. E. Anterior (Dor) fundoplication is sutured to the diaphragmatic arch. F. Posterior (Toupet) fundoplication is sutured to cut edges of myotomy. EG jct = esophagogastric junction. 1067 CHAPTER 25 ESOPHAGUS AND DIAPHRAGMATIC HERNIA Figure 25-65. (Continued ) Table 25-10 Reasons for failure of esophageal myotomy AUTHOR, PROCEDURE (N) REASON ELLIS, MYOTOMY ONLY (N = 81) GOULBOURNE, MYOTOMY ONLY (N = 65) MALTHANER, MYOTOMY + ANTIREFLUX (N = 22) Reflux 4% 5% 18% Inadequate myotomy 2% — 9% Megaesophagus 2% — — Poor emptying 4% 3% — Persistent chest pain 1% — — Data from Malthaner RA, et al. Long-term results in surgically managed esophageal achalasia. Ann Thorac Surg. 1994;58:1343; Ellis FH, Jr. Oesophagomyotomy for achalasia: a 22-year experience. Br J Surg. 1993;80:882; and Goulbourne IA, et al. Long-term results of Heller’s operation for achalasia. J R Coll Surg Edinb. 1985;30:101. 1068 PART II SPECIFIC CONSIDERATIONS The outcome of laparoscopic myotomy and hemifundoplication has been well documented. Two reports of over 100 patients have documented relief of dysphagia in 93% of patients. Richter and coworkers reviewed published reports to date, including 254 patients with an average success rate of 93% at 2.5 years. Conversion to an open procedure occurs in 0% to 5% of patients. Complications are uncommon, occurring in <5% of patients. Intraoperative complications consist largely of mucosal perforation, and have been more likely to occur after botulinum toxin injection. The incidence of objective reflux disease as evidenced by abnormal acid exposure is <10%. A number of randomized clinical trials in the past decade have compared the outcomes of laparoscopic Heller myotomy to pneumatic dilation and to botulinum toxin injection. In each of these trials, laparoscopic Heller myotomy and partial fundoplication was superior to the alternative treatment. Lastly, a randomized clinical trial examining the need for fundoplication following Heller myotomy demonstrated a great deal more reflux in patients without fundoplication, and no better swallowing in the Heller-only group. The best treatment for achalasia is a laparoscopic Heller myotomy and partial fundoplication. The role of POEM in the management of classic (nonspastic) achalasia is yet to be established. Esophageal Resection for End-Stage Motor Disorders of the Esophagus Patients with dysphagia and long-standing benign disease, whose esophageal function has been destroyed by the disease process or multiple previous surgical procedures, are best managed by esophagectomy. Fibrosis of the esophagus and cardia can result in weak contractions and failure of the distal esophageal sphincter to relax. The loss of esophageal contractions can result in the stasis of food, esophageal dilatation, regurgitation, and aspiration. The presence of these abnormalities signals endstage motor disease. In these situations, esophageal replacement is usually required to establish normal alimentation. Before proceeding with esophageal resection for patients with end-stage benign disease, the choice of the organ to substitute for the esophagus (i.e., stomach, jejunum, or colon) should be considered. The choice of replacement is affected by a number of factors, as described later in “Techniques of Esophageal Reconstruction.” If minimally invasive esophagectomy is to be performed, thoracoscopic dissection should be combined with abdominal dissection. Attempts at MIS transhiatal esophagectomy for the massively dilated esophagus may result in large volume bleeding from mediastinal vessels that become enlarged with esophageal dilation, and such bleeding must be directly controlled for hemostasis to be adequate and the operation to be safe. CARCINOMA OF THE ESOPHAGUS Squamous carcinoma accounts for the majority of esophageal carcinomas worldwide. Its incidence is highly variable, ranging from approximately 20 per 100,000 in the United States and Britain, to 160 per 100,000 in certain parts of South Africa and the Henan Province of China, and even 540 per 100,000 in the Guriev district of Kazakhstan. The environmental factors responsible for these localized high-incidence areas have not been conclusively identified, though additives to local foodstuffs (nitroso compounds in pickled vegetables and smoked meats) and mineral deficiencies (zinc and molybdenum) have been suggested. In Western societies, smoking and alcohol consumption are strongly linked with squamous carcinoma. Other definite associations link squamous carcinoma with long-standing achalasia, lye strictures, tylosis (an autosomal dominant disorder characterized by hyperkeratosis of the palms and soles), and human papillomavirus. Adenocarcinoma of the esophagus, once an unusual malignancy, is diagnosed with increasing frequency (Fig. 25-66) and now accounts for more than 50% of esophageal cancer in most Western countries. The shift in the epidemiology of esophageal cancer from predominantly squamous carcinoma seen in association with smoking and alcohol to adenocarcinoma in the setting of BE is one of the most dramatic changes that has occurred in the history of human neoplasia. Although esophageal carcinoma is a relatively uncommon malignancy, its prevalence is exploding, largely secondary to the well-established association among gastroesophageal reflux, BE, and esophageal adenocarcinoma. Although BE was once a nearly uniformly lethal disease, survival has improved slightly because of advances in the understanding of its molecular biology, screening and surveillance practices, improved staging, minimally invasive surgical techniques, and neoadjuvant therapy. Furthermore, the clinical picture of esophageal adenocarcinoma is changing. It now occurs not only considerably more frequently but also in younger patients, and it is often detected at an earlier stage. These facts support rethinking the traditional approach of assuming palliation is appropriate in all patients. The historical focus on palliation of dysphagia in an elderly patient with comorbidities should change when dealing with a young patient with dependent children and a productive life ahead. The potential for cure becomes of paramount importance. The gross appearance resembles that of squamous cell carcinoma. Microscopically, adenocarcinoma almost always originates in Barrett’s mucosa and resembles gastric cancer. Rarely, it arises in the submucosal glands and forms intramural growths that resemble the mucoepidermal and adenoid cystic carcinomas of the salivary glands. The most important etiologic factor in the development of primary adenocarcinoma of the esophagus is a metaplastic columnar-lined or Barrett’s esophagus, which occurs in approximately 10% to 15% of patients with GERD. When studied prospectively, the incidence of adenocarcinoma in a patient with BE is one in 100 to 200 patient-years of follow-up (i.e., for every 100 patients with BE followed for 1 year, one will develop adenocarcinoma). Although this risk appears to be small, it is at least 40 to 60 times that expected for a similar population without BE. This risk is similar to the risk for developing lung cancer in a person with a 20-pack-per-year history of smoking. Endoscopic surveillance for patients with BE is recommended for two reasons: (a) at present there is no reliable evidence that medical therapy removes the risk of neoplastic transformation, and (b) malignancy in BE is curable if detected at an early stage. Clinical Manifestations Esophageal cancer generally presents with dysphagia, although increasing numbers of relatively asymptomatic patients are now identified on surveillance endoscopy, or present with nonspecific upper GI symptoms and undergo screening Extension of the primary tumor into the 6 endoscopy. tracheobronchial tree can occur primarily with squamous cell carcinoma and can cause stridor, tracheoesophageal fistula, and resultant coughing, choking, and aspiration U.S. esophageal cancer incidence 20 15 10 5 0 1985 1993 1989 1997 2001 2005 U.S. esophageal cancer mortality 25 Mortality per 100,000 20 15 10 General Approach to Esophageal Cancer 5 0 1985 1993 1989 1997 2001 2005 White females Overall rate White males African American males African American females NCI esophageal cancer research investment $4.6B $21.8M $4.7B $21.7M $4.8B $22.7M $4.7B $21.6M $4.8B $22.3M 4 20 Millions of dollars 5 3 15 10 2 5 1 0 2003 2004 2005 2006 2007 Billions of dollars 25 pneumonia. Rarely, severe bleeding from the primary tumor or from erosion into the aorta or pulmonary vessels occurs. Either vocal cord may be invaded, causing paralysis, but most commonly, paralysis is caused by invasion of the left recurrent laryngeal nerve by the primary tumor or LN metastasis. Systemic organ metastases are usually manifested by jaundice or bone pain. The situation is different in high-incidence areas where screening is practiced. In these communities, the most prominent early symptom is pain on swallowing rough or dry food. In patients that present with back pain at the time of esophageal cancer diagnosis, there is usually distant metastasis or celiac encasement. Dysphagia usually presents late in the natural history of the disease because the lack of a serosal layer on the esophagus allows the smooth muscle to dilate with ease. As a result, the dysphagia becomes severe enough for the patient to seek medical advice only when more than 60% of the esophageal circumference is infiltrated with cancer. Consequently, the disease is usually advanced if symptoms herald its presence. Tracheoesophageal fistula may be present in some patients on their first visit to the hospital, and more than 40% will have evidence of distant metastases. With tumors of the cardia, anorexia and weight loss usually precede the onset of dysphagia. The physical signs of esophageal tumors are those associated with the presence of distant metastases. 0 Fiscal year Esophageal cancer funding Total NCI budget Figure 25-66. Incidence and mortality rate trends for esophageal cancer. NCI = National Cancer Institute. (Reproduced with permission from the National Cancer Institute. Last updated September, 2008.) Therapy of esophageal cancer is dictated by the stage of the cancer at the time of diagnosis. Put simply, one needs to determine if the disease is confined to the esophagus, (T1–T2, N0), locally advanced (T1–3, N1), or disseminated (any T, any N, M1). If cancer is confined to the esophagus, removal of the tumor with adjacent lymph nodes may be curative. Very early tumors confined to the mucosa (T in situ, T1a, intramucosal cancer) may be addressed with endoscopic treatment. When the tumor is locally aggressive, modern therapy dictates a multimodality approach in a surgically fit patient. Multimodality therapy is either chemotherapy followed by surgery or radiation and chemotherapy followed by surgery. When given before surgery, these treatments are referred to as neoadjuvant or induction therapy. For disseminated cancer, treatment is aimed at palliation of symptoms. If the patient has dysphagia, as many do, the most rapid form of palliation is the endoscopic placement of an expandable esophageal stent. For palliation of GEJ cancer, radiation may be the first choice, as stents placed across the GEJ create a great deal of gastroesophageal reflux. Staging of Esophageal Cancer Choosing the best therapy for an individual patient requires accurate staging. Staging starts with the history and physical. LN disease remote from the tumor, particularly in the cervical region, may be palpable on neck examination and generally indicates cancer dissemination. This is often referred to as M1a disease, indicating that these patients should not be treated with therapy directed toward locally advanced cancer. Other metastatic LNs are rarely palpable but are equally ominous, especially the umbilical LN in GEJ cancer. Computed tomographic (CT) scanning of the chest, abdomen, and pelvis provides information on local invasion of the primary cancer, LN involvement, or disseminated disease. The most common sites of esophageal cancer metastases are lung, liver, and peritoneal surfaces, including the omentum and small bowel mesentery. If masses are identified that are 1069 CHAPTER 25 ESOPHAGUS AND DIAPHRAGMATIC HERNIA Incidence per 100,000 25 1070 PART II SPECIFIC CONSIDERATIONS not characteristic for cancer or are in a location that precludes resection with the cancer specimen, positron emission tomography (PET) scanning may be able to tell whether the masses are metabolically active (likely to be cancer) or not. A PET active focus corresponding to a mass on CT scan outside of the field of esophageal resection should be biopsied before resection is performed. The introduction of endoscopic ultrasound (EUS) has made it possible to identify patients who are potentially curable before surgical therapy. Using an endoscope, the depth of the wall penetration by the tumor and the presence of LN metastases can be determined with 80% accuracy. A curative resection should be encouraged if EUS indicates that the tumor has not invaded adjacent organs (T4b), and/or fewer than six enlarged LNs are imaged. Thoracoscopic and laparoscopic staging of esophageal cancer may add benefit when the nature of enlarged LNs remote from the cancer cannot be determined or when advanced imaging systems (PET and high-resolution spiral CT) are not available. Occasionally, diagnostic laparoscopy and jejunostomy tube placement may precede induction chemoradiation in the patient with severe dysphagia and weight loss from a locally advanced cancer. In summary, esophageal cancer is diagnosed with endoscopic biopsy and is staged with CT scanning of the chest and abdomen, EUS, and PET scan for all patients with CT or EUS evidence of advanced disease (T2 or greater, N1-2 or NX). Experience with esophageal resection in patients with early stage disease has identified characteristics of esophageal cancer that are associated with improved survival. A number of studies suggest that only metastasis to LNs and tumor penetration of the esophageal wall have a significant and independent influence on prognosis. Factors known to be important in the survival of patients with advanced disease, such as cell type, degree of cellular differentiation, or location of tumor in the esophagus, have no effect on survival of patients who have undergone resection for early disease. Studies also showed that patients having five or fewer LN metastases have a better outcome. Using these data, Skinner developed the wall penetration, LN, and distant organ metastases system for staging. The wall penetration, LN, and distant organ metastases system differed somewhat from the previous efforts to develop a satisfactory staging criteria for carcinoma of the esophagus. Most surgeons agreed that the 1983 tumor, nodes, and metastasis system left much to be desired. In the third edition of the manual for Staging of Cancer of the American Joint Committee on Cancer (AJCC) in 1988, an effort was made to provide a finer discrimination between stages than had been contained in the previous edition in 1983. In 2016, further refinements of the staging system of esophageal cancer were approved by the AJCC, recognizing the difference in survival afforded by resection of limited LN disease adjacent to the tumor, compared to multilevel LN disease and positive LNs remote from the primary. Table 25-11 shows the AJCC definitions for the primary tumor, lymph nodes, distant metastasis, and overall staging schema for both squamous cell carcinoma and adenocarcinoma. Clinical Approach to Carcinoma of the Esophagus and Cardia The selection of a curative vs. a palliative operation for cancer of the esophagus is based on the location of the tumor, the patient’s age and health, the extent of the disease, and preoperative staging. Figure 25-67 shows an algorithm of the clinical decisions important in the selection of curative or palliative therapy. Tumor Location. The selection of surgical therapy for patients with carcinoma of the esophagus depends not only on the anatomic stage of the disease and an assessment of the swallowing capacity of the patient but also on the location of the primary tumor. It is estimated that 8% of the primary malignant tumors of the esophagus occur in the cervical portion (Fig. 25-68). They are almost always squamous cell cancer, with a rare adenocarcinoma arising from a congenital inlet patch of columnar lining. These tumors, particularly those in the postcricoid area, represent a separate pathologic entity for two reasons: (a) they are more common in females and appear to be a unique entity in this regard; and (b) the efferent lymphatics from the cervical esophagus drain completely differently from those of the thoracic esophagus. The latter drain directly into the paratracheal and deep cervical or internal jugular LNs with minimal flow in a longitudinal direction. Except in advanced disease, it is unusual for intrathoracic LNs to be involved. Cervical esophageal cancer is frequently unresectable because of early invasion of the larynx, great vessels, or trachea. Radical surgery, including esophagolaryngectomy may occasionally be performed for these lesions, but the ensuing morbidity makes this a less than desirable approach in the face of uncertain cure. Thus, for most patients with cervical esophageal cancer, stereotactic radiation with concomitant chemotherapy is the most desirable treatment. Tumors that arise within the middle third of the esophagus are squamous carcinomas most commonly and are frequently associated with LN metastasis, which are usually in the thorax but may be in the neck or abdomen, and may skip areas in between. Although it is generally felt that individuals with midthoracic cancer and abdominal LN metastases are incurable with surgery, there are some emerging data that suggest that cervical LN metastases, if isolated, can be resected with benefit. Generally, T1 and T2 cancers without LN metastases are treated with resection only, but there is more and more data to suggest that LN involvement or transmural cancer (T3) warrants treatment with neoadjuvant chemoradiation therapy followed by resection. Although some surgeons prefer a transhiatal esophagectomy for all tumor locations, most surgeons believe that resection of midesophageal cancer should be performed under direct vision with either thoracoscopy (video-assisted thoracic surgery [VATS]) or with thoracotomy. Tumors of the lower esophagus and cardia are usually adenocarcinomas. Unless preoperative and intraoperative staging clearly demonstrate an incurable lesion, resection in continuity with a LN dissection should be performed. Because of the propensity of GI tumors to spread for long distances submucosally, long lengths of grossly normal GI tract should be resected. The longitudinal lymph flow in the esophagus can result in skip areas, with small foci of tumor above the primary lesion, which underscores the importance of a wide resection of esophageal tumors. Wong has shown that local recurrence at the anastomosis can be prevented by obtaining a 10-cm margin of normal esophagus above the tumor. Anatomic studies have also shown that there is no submucosal lymphatic barrier between the esophagus and the stomach at the cardia, and Wong has 1071 Table 25-11 American Joint Committee on Cancer (AJCC) Staging Schema for Esophageal Cancer Primary tumor cannot be assessed. No evidence of primary tumor. High-grade dysplasia. Tumor invades lamina propria, muscularis mucosae, or submucosa. Tumor invades lamina propria or muscularis mucosae. Tumor invades submucosa. Tumor invades muscularis propria. Tumor invades adventitia. Tumor invades adjacent structures. Resectable tumor invading pleura, pericardium, or diaphragm. Unresectable tumor invading other adjacent structures, such as aorta, vertebral body, trachea, etc. Regional lymph nodes cannot be assessed. No regional lymph node metastasis. Metastases in 1–2 regional lymph nodes. Metastases in 3–6 regional lymph nodes. Metastases in ≥7 regional lymph nodes. No distant metastasis. Distant metastasis. SQUAMOUS CELL CARCINOMA Clinical (cTNM) When cT is... Tis T1 T2 T3 T3 T1–3 T4 Any T Any T And cN is... N0 N0–1 N0–1 N0 N1 N2 N0–2 N3 Any N Pathological (pTNM) When And And And pT is... pN is... M is... G is... Tis N0 M0 N/A T1a N0 M0 G1 T1a N0 M0 G2–3 T1a N0 M0 GX T1b N0 M0 G1–3 T1b N0 M0 GX T2 N0 M0 G1 T2 N0 M0 G2–3 T2 N0 M0 GX T3 N0 M0 G1–3 T3 N0 M0 G1 T3 N0 M0 G2–3 And M is... M0 M0 M0 M0 M0 M0 M0 M0 M1 Then the stage group is... 0 I II II III III IVA IVA IVB T3 T3 T1 T1 T2 T2 T3 T4a T4a T4b Any T Any T N0 M0 N0 M0 N1 M0 N2 M0 N1 M0 N2 M0 N1–2 M0 N0–1 M0 N2 M0 N0–2 M0 N3 M0 Any N M1 GX Any Any Any Any Any Any Any Any Any Any Any Lower/upper/middle IIB Location X IIB Any IIB Any IIIA Any IIIA Any IIIB Any IIIB Any IIIB Any IVA Any IVA Any IVA Any IVB Postneoadjuvant Therapy (ypTNM) And location is... Any Any Any Any Any Any Any Any Any Lower Upper/middle Upper/middle Then the stage group is... 0 IA IB IA IB IB IB IIA IIA IIA IIA IIB When yp T is... T0–2 T3 T0–2 T3 T0–3 T4a T4a T4a T4b Any T Any T And yp N is... N0 N0 N1 N1 N2 N0 N1–2 NX N0–2 N3 Any N And M is... M0 M0 M0 M0 M0 M0 M0 M0 M0 M0 M1 Then the stage group is... I II IIIA IIIB IIIB IIIB IVA IVA IVA IVA IVB ADENOCARCINOMA Clinical (cTNM) When And cT is... cN is... Tis N0 T1 N0 T1 N1 T2 N0 And M is... M0 M0 M0 M0 Then the stage group is... 0 I IIA IIB T2 T3 T4a T1–4a T4b Any T Any T N1 N0–1 N0–1 N2 N0–2 N3 Any N M0 M0 M0 M0 M0 M0 M1 III III III IVA IVA IVA IVB (Continued) CHAPTER 25 ESOPHAGUS AND DIAPHRAGMATIC HERNIA TX T0 Tis T1 T1a T1b T2 T3 T4 T4a T4b NX N0 N1 N2 N3 M0 M1 1072 Table 25-11 American Joint Committee on Cancer (AJCC) Staging Schema for Esophageal Cancer (Continued) Pathological (pTNM) PART II SPECIFIC CONSIDERATIONS When pT is... Tis T1a T1a T1a T1b T1b T1 T2 T2 T2 T1 T3 T1 T2 T2 T3 And pN is... N0 N0 N0 N0 N0 N0 N0 N0 N0 N0 N1 N0 N2 N1 N2 N1–2 And M is... M0 M0 M0 M0 M0 M0 M0 M0 M0 M0 M0 M0 M0 M0 M0 M0 And G is... N/A G1 GX G2 G1–2 GX G3 G1–2 G3 GX Any Any Any Any Any Any Then the stage group is... 0 IA IA IB IB IB IC IC IIA IIA IIB IIB IIIA IIIA IIIB IIIB T4a T4a T4b Any T Any T N0–1 N2 N0–2 N3 Any N M0 M0 M0 M0 M1 Postneoadjuvant Therapy (ypTNM) When yp And yp And T is... N is... M is... T0–2 N0 M0 T3 N0 M0 T0–2 N1 M0 T3 N1 M0 T0–3 N2 M0 T4a N0 M0 T4a N1–2 M0 T4a NX M0 T4b N0–2 M0 Any T N3 M0 Any T Any N M1 Any Any Any Any Any IIIB IVA IVA IVA IVB Then the stage group is... I II IIIA IIIB IIIB IIIB IVA IVA IVA IVA IVB Used with the permission of the American College of Surgeons. Amin MB, Edge SB, Greene FL, et al. (Eds.) AJCC Cancer Staging Manual, 8th Ed. Springer New York, 2017. shown that 50% of the local recurrences in patients with esophageal cancer who are resected for cure occur in the intrathoracic stomach along the line of the gastric resection. Considering that the length of the esophagus ranges from 17 to 25 cm, and the length of the lesser curvature of the stomach is approximately 12 cm, a curative resection requires a cervical division of the esophagus and a >50% proximal gastrectomy in most patients with carcinoma of the distal esophagus or cardia. Age Physiologic fitness Clinical staging patient older than 80 years is rarely indicated because of the additional operative risk and the shorter life expectancy. Despite this general guideline, octogenarians with a high-performance status and excellent cardiopulmonary reserve may be considered candidates for esophagectomy, and recent case series have established its success in highly selected patients. It is in this group of patients that the lesser physiologic impact of minimally Palliation 75 years Palliation FEV1 1.25 Ejection fraction 40% Palliation Recurrent nerve paralysis Horner's syndrome Persistent spinal pain Paralysis of diaphragm Fistula formation Malignant pleural effusion Endoscopic tumor length 9 cm Abnormal esophageal axis Multiple enlarged nodes or distant organ metastasis on CT More than 20% weight loss Loss of appetite (relative) Endoscopic ultrasound Palliation Transmural tumors with 4 enlarged nodes Intraoperative staging Palliation Unresectable primary Cavitary spread Distant metastasis Extension through mediastinal wall Multiple gross lymph node metastases Microscopic nodal metastasis at margins of the en bloc dissection Curative en bloc resection Age. Resection for cure of carcinoma of the esophagus in a Palliative symptoms Dysphagia Obstruction Pain of ulceration Bleeding Infection Anxiety Requirements for palliative transhiatal resection* - Free of distant organ metastases - Complete excision of primary tumor possible Nonsurgical palliation *Could include combined Rx and chemo neoadjuvant therapy prior to resection to increase resectability and potential survival in patients 75 or under. Figure 25-67. Algorithm for the evaluation of esophageal cancer patients to select the proper therapy: curative en bloc resection, palliative transhiatal resection, or nonsurgical palliation. CT = computed tomography; FEV1 = forced expiratory volume in 1 second. (Reproduced with permission from DeMeester TR: Esophageal carcinoma: current controversies, Semin Surg Oncol. 1997 Jul-Aug;13(4):217-233.) Location Incidence Cervical 8% Upper thoracic 3% 32% Lower thoracic 25% Cardia 32% Figure 25-68. Incidence of carcinoma of the esophagus and cardia based on tumor location. invasive surgery may reduce the morbidity and mortality associated with open two- or three-field esophagectomy. Cardiopulmonary Reserve. Patients undergoing esophageal resection should have sufficient cardiopulmonary reserve to tolerate the proposed procedure. The respiratory function is best assessed with the forced expiratory volume in 1 second, which ideally should be 2 L or more. Any patient with a forced expiratory volume in 1 second of <1.25 L is a poor candidate for thoracotomy because he or she has a 40% risk of dying from respiratory insufficiency within 4 years. In patients with poor pulmonary reserve, the transhiatal esophagectomy should be considered, as the pulmonary morbidity of this operation is less than is seen following thoracotomy. Clinical evaluation and electrocardiogram are not sufficient indicators of cardiac reserve. Echocardiography and dipyridamole thallium imaging provide accurate information on wall motion, ejection fraction, and myocardial blood flow. A defect on thallium imaging may require further evaluation with preoperative coronary angiography. A resting ejection fraction of <40%, particularly if there is no increase with exercise, is an ominous sign. In the absence of invasive testing, observed stair-climbing is an economical (albeit not quantitative) method of assessing cardiopulmonary reserve. Most individuals who can climb three flights of stairs without stopping will do well with two-field open esophagectomy, especially if an epidural catheter is used for postoperative pain relief. Nutritional Status. The factor most predictive of postoperative complication is the nutritional status of the patient. Profound weight loss, more than 20 lb, associated with hypoalbuminemia (albumin <3.5 g/dL) is associated with a much higher rate of complications and mortality than patients who enter curative surgery in better nutritional condition. Because malnourished patients generally have locally advanced esophageal cancer, if not metastatic disease, one should consider the placement of a feeding tube before the beginning of induction chemoradiation therapy. Although mild amounts of dysphagia are improved by 1073 Clinical Staging. Clinical factors that indicate an advanced stage of carcinoma and exclude surgery with curative intent are recurrent nerve paralysis, Horner’s syndrome, persistent spinal pain, paralysis of the diaphragm, fistula formation, and malignant pleural effusion. Factors that make surgical cure unlikely include a tumor >8 cm in length, abnormal axis of the esophagus on a barium radiogram, more than four enlarged LNs on CT, a weight loss more than 20%, and loss of appetite. Studies indicate that there are several favorable parameters associated with tumors <4 cm in length, there are fewer with tumors between 4 and 8 cm, and there are no favorable criteria for tumors >8 cm in length. Consequently, the finding of a tumor >8 cm in length should exclude curative resection; the finding of a smaller tumor should encourage an aggressive approach. Preoperative Staging With Advanced Imaging. For years, clinical staging, contrast radiography, endoscopy, and CT scanning formed the backbone of esophageal cancer staging. More recently, preoperative decision making is guided by endoscopic ultrasonography and PET scanning. EUS provides the most reliable method of determining depth of cancer invasion. In the absence of enlarged LNs, the degree of wall invasion dictates surgical therapy. If a small focus of esophageal cancer is confined to the mucosa, endoscopic mucosal resection (EMR) is a preferable option. If the tumor invades into the submucosa, without visible lymph node involvement, most individuals would suggest esophagectomy with LN dissection, as positive nodes can be found in 20% to 25% of those with cancer limited to the mucosa and submucosa. If EUS demonstrates spread through the wall of the esophagus, especially if LNs are enlarged, then induction chemoradiation therapy (neoadjuvant therapy) should be strongly considered. Lastly, when the EUS demonstrates invasion of the trachea, bronchus, aorta, or spine, then surgical resection is rarely indicated. If there is invasion into the pleura (T4a), then surgical resection can be considered in the absence of a malignant effusion. Thus, it can be seen that the therapy of esophageal cancer is largely driven by the findings of an endoscopic ultrasonography. It is difficult to provide modern treatment of esophageal cancer without access to this modality. PET scanning, usually combined with an axial CT scan (CTPET), usually is performed on patients with locally advanced cancer or questionable lesions on CT scan to determine whether metastases are present. The PET scan uses the injection of radiolabeled deoxyglucose, which is taken up in metabolically active tissues such as cancer. PET-positive areas must be correlated with the CT scan findings to assess the significance of “hot spots.” CTPET scanning has been especially useful before the initiation of chemoradiation therapy. An early response to chemoradiotherapy, by PET scan, improves the prognosis whether or not resection is ultimately performed. Conversely, if a PET-avid tumor shows no change in metabolic activity after 2 weeks of induction chemoradiation therapy, it is unlikely that further chemo- or radiation therapy will be of CHAPTER 25 ESOPHAGUS AND DIAPHRAGMATIC HERNIA Middle thoracic induction chemoradiation therapy, more pronounced dysphagia and associated malnutrition should be addressed before the initiation of chemoradiation. A laparoscopic jejunostomy tube can be placed prior to induction therapy or at the time of esophagectomy. There are emerging data that 5 days’ pretreatment with immune-enhancing nutrition, rich in fish oils, decreases cardiac and other complications, following esophagectomy. 1074 any benefit. These patients have a worse prognosis and may be referred for resection or palliation without incurring the morbidity or expense of a full course of chemo- and radiation therapy. Palliation of Esophageal Cancer PART II SPECIFIC CONSIDERATIONS Palliation of esophageal cancer is indicated for individuals with metastatic esophageal cancer or cancer invading adjacent organs (T4b) who are unable to swallow, or individuals with fistulae into the tracheobronchial tree. Aortic esophageal fistulas are extremely rare and nearly 100% lethal. Dysphagia as a result of esophageal cancer can be graded from grade I, eating normally, to grade VI, unable to swallow saliva (Table 25-12). Grades I to III often can be managed with radiation therapy, usually in combination with chemotherapy. When surgical resection is not anticipated in the future, this is termed definitive chemoradiation therapy and usually is palliative. Radiation dose is increased from 45 Gy to 60 Gy administered over 8 weeks, rather than the 4 weeks given for chemoradiation induction therapy. In 20% of patients, a complete response to chemoradiation therapy will not only palliate the symptoms but will also leave the patient with undetectable cancer of the esophagus. Although some of these patients are truly cured, cancer will recur in many either locally or systemically 1 to 5 years following definitive chemoradiation. In a few patients, definitive chemoradiation will be successful in all sites but the esophagus. After a 12-month wait from initial treatment and no other sites of tumor detectable except the esophagus, some of these patients may be candidates for salvage esophagectomy. For individuals with dysphagia grades IV and higher, additional treatment generally is necessary. The mainstay of therapy is in-dwelling esophageal stents. Covered removable stents may be used to seal fistulae or when stent removal becomes desirable in the future. When large, locally invasive tumors or metastatic esophageal cancer precludes any future hope of resection, uncovered expandable metal stents are the treatment of choice. The major limitations to stenting exist in cancers at the GEJ. A stent placed across the GEJ will result in severe gastroesophageal reflux and heartburn that can be quite disabling. In cancers at this level, radiation therapy alone may be preferable. If feeding access is desirable, a laparoscopic jejunostomy is usually the procedure of choice. Table 25-12 Functional grades of dysphagia GRADE DEFINITION INCIDENCE AT DIAGNOSIS (%) I Eating normally 11 II Requires liquids with meals 21 III Able to take semisolids but unable to take any solid food 30 IV Able to take liquids only 40 V Unable to take liquids, but able to swallow saliva 7 VI Unable to swallow saliva 12 Data from Takita H, Vincent RG, Caicedo V, et al. Squamous cell carcinoma of the esophagus: a study of 153 cases, J Surg Oncol. 1977;9(6):547-554. Surgical Treatment The surgical treatment of esophageal cancer is dependent upon the location of the cancer, the depth of invasion, LN metastases, the fitness of the patient for operation, and the culture and beliefs of the individuals and institutions in which the treatment is performed. In an ideal world, there would be a single, stage-specific method of treating esophageal cancer because the evidence would be unassailable and noncontroversial. Randomized clinical trials and meta-analyses would prove beyond a shadow of a doubt the value of surgery vs. nonoperative therapy and would dictate the type and extent of surgery that would optimally balance immediate morbidity and mortality with duration and quality of life conferred by the procedure and the perioperative management of the esophagectomy patient. Despite many noble attempts to establish this high level of evidence, many questions relating to the appropriate therapy of esophageal cancer remain. About the only area of complete agreement is that esophagectomy should not be performed if an R0 resection is not possible. In other words, if the surgeon does not believe he or she can remove all LNs invaded by cancer and provide a tumor-free radial margin and esophagus and stomach margins that are tumor free, then a resection should not be performed. Mucosally Based Cancer. In patients with BE, and especially those with high-grade dysplasia, subcentimeter nodules are frequently discovered. Nodules should be resected in entirety, as they often harbor adenocarcinoma. Five years ago, such resection was performed with a transhiatal esophagectomy, but more recently EMR offers another method for removing intramucosal cancer. In this clinical situation, EMR is typically combined with EUS to rule out more invasive disease. EUS, however, is unable to differentiate between cancer that is confined to the mucosa (T1a) and that which invades the submucosa (T1b). Tumors invading the submucosa are not amenable to endoscopic mucosal resection because of the high-frequency (20–25%) concurrent finding of positive LNs, which cannot be removed without esophagectomy. On the other hand, intramucosal cancers have little risk of spreading to regional LNs. The current approach used involves performing EMR on all nodules identified in a field of Barrett’s esophagus, and then T staging is performed by histologic analysis. This approach dictates the need for future therapy such as esophagectomy. For this reason, small intramucosal carcinomas may be removed with EMR in the following manner. The area beneath the nodule is infiltrated with saline through a sclerotherapy needle. A specialized suction cap is mounted on the end of the endoscope, and the nodule is drawn up into the cap; a snare is then applied to resect the tissue. Alternatively, a rubber band can be delivered, and the snare can be used to resect above the level of the rubber band. This specimen is then removed and sent to pathology. As long as the tumor is found to be confined to the mucosa and all margins are negative, the resection is complete. A positive margin or involvement of the submucosa warrants esophagectomy. Most importantly, these patients are at high risk for developing small nodular carcinomas elsewhere in their Barrett’s segment, and routine surveillance on a 3- to 6-month basis must be continued indefinitely. Alternatively, one can consider radiofrequency ablation of the remainder of the high-grade dysplasia after careful surveillance biopsy specimens demonstrate no further sign of cancer. This approach to the early esophageal cancer 1075 should not be used when there is any suspicion of mediastinal or abdominal lymphadenopathy. Although it is currently rare that EMR provides definitive therapy of small nodular esophageal cancers, this may become more of the norm as greater surveillance reveals earlier cancers and proficiency of the technique by surgeons and gastroenterologists increases. Minimally Invasive Transhiatal Esophagectomy. CHAPTER 25 ESOPHAGUS AND DIAPHRAGMATIC HERNIA Minimally invasive transhiatal esophagectomy is an increasingly popular procedure; however, the number of these operations performed around the world remains small. Mini-invasive surgery (MIS) transhiatal esophagectomy was first performed by Aureo DePaula in Brazil and has been modified and adopted by many individuals around the world. This operation combines the advantages of transhiatal esophagectomy at minimizing pulmonary complications with the advantages of laparoscopy (less pain, quicker rehabilitation). Several variations of MIS transhiatal esophagectomy have been developed. For the earliest lesions, such as high-grade dysplasia or intramucosal carcinoma, a vagal sparing procedure can be entertained. In such a procedure, the vagal trunks are separated from the esophagus at the level of the diaphragm and the lesser curvature dissection of the stomach allows the vagus and left gastric pedicle to remain intact. Clearly, this dissection, which hugs the stomach and esophagus, provides no LN staging and is thus inadequate for all high-grade dysplasia and intramucosal cancer. MIS transhiatal esophagectomy is usually performed through five or six small incisions in the upper abdomen and a transverse cervical incision for removing the specimen and performing the cervical esophagogastrostomy. To remove the esophagus from the posterior mediastinum, especially the area behind the pulmonary vessels and the tracheal bifurcation, which cannot be visualized even with a long laparoscope placed in the posterior mediastinum, it is preferred to use a vein stripping “inversion” technique (Fig. 25-69A). The details of this operation are too lengthy to include in this text, but include the laparoscopic creation of a neo-esophagus (gastric conduit) along the greater curvature of the stomach using the right gastroepiploic artery as the primary vascular pedicle. The conduit can be created through a mini-laparotomy or laparoscopically. A Kocher maneuver releases the duodenum, and a pyloroplasty may be performed (optional). Retrograde esophageal stripping is performed by dividing the esophagus below the GEJ and sliding a vein stripper from the neck down into the abdomen followed by an inversion of the esophagus in the posterior mediastinum and removal through the neck (Fig. 25-69B). This technique is reserved for patients with high-grade dysplasia. For small cancers at the GEJ, the esophagus can be stripped in an antegrade fashion by sliding the vein stripper down from the cervical incision and out the tail of the lesser curvature (Fig. 25-69C). The tail of the lesser curvature is pulled out a port site high in the epigastrium while the esophagus is inverted into itself. For GEJ cancers, a wide celiac access LN dissection, splenic artery, hepatic artery, and posterior mediastinal LN dissection can be performed as well or better than through a laparotomy. The gastric conduit is pulled up to the neck with a chest tube and anastomosed to the cervical esophagus in an end-to-side fashion using a surgical stapler or with a handsewn anastomosis. Complications of this technique are primarily limited to leak from the esophagogastric anastomosis, which is self-limited and usually heals within 1 to 3 weeks, spontaneously. Figure 25-69. A. Laparoscopic retrograde inversion. B. Laparoscopic antegrade inversion. A silk suture holds the tunnel after the esophagus is removed. C. The esophageal conduit is returned to the neck after passing a chest tube down the tunnel and suturing the conduit to the chest tube. 1076 PART II SPECIFIC CONSIDERATIONS Open Transhiatal Esophagectomy. Transhiatal esophagectomy, also known as blunt esophagectomy or esophagectomy without a thoracotomy, was first performed in 1933 by a British surgeon, but was popularized in the last quarter of the 20th century by Mark Orringer from the University of Michigan. Although this operation may violate many of the principles of cancer resection, including extended radical LN dissection, this operation has performed as well as any of the more radical procedures in randomized trials, and in large database analyses. With transhiatal esophagectomy, the elements of dissection are similar to that described in the section entitled Minimally Invasive Transhiatal Esophagectomy, including the creation of the gastric tube and the posterior mediastinal dissection through the hiatus. Because this dissection is performed with the fingertips rather than under direct vision with surgical instruments, it requires an enlargement of the diaphragmatic hiatus. The lower mediastinal LN basins can be resected as can the upper abdominal LNs, making this an attractive option for GEJ cancers. The mediastinal LNs above the inferior pulmonary vein are not removed with this technique, but they rarely result in a point of isolated cancer recurrence. Of all procedures for esophageal cancer, this operation is the quickest to perform in experienced hands and lies in an intermediate position between minimally invasive esophagectomy and the Ivor Lewis procedure with respect to complications and recovery. Minimally Invasive Two- and Three-Field Esophagectomy. After a rocky start, minimally invasive esophagectomy using a thoracic dissection through VATS has become reasonably popular. In general, this operation is performed with an anastomosis created in the neck (three-field), but it may be performed with the anastomosis stapled in the high thorax (twofield). Both procedures will be described. With a minimally invasive three-field esophagectomy, the patient is placed in the left lateral decubitus position. Double lumen intubation is required. Videoscopic access to the thorax is obtained in the midaxillary line in the ninth intercostal space and an angled telescope illuminates the chest superiorly. A mini-thoracotomy at about the sixth intercostal space anteriorly allows introduction of conventional surgical instruments, and a high trocar allows retraction of the lung away from the esophagus. In a three-field approach, the esophagus is dissected along its length to include division of the azygos vein and harvesting of the LNs in the upper, middle, and lower posterior mediastinum. Hilar, and posterior mediastinal nodes are all removed and sent with the specimen or individually. The thoracic duct is divided at the level of the diaphragm and removed with the specimen. Following complete intrathoracic dissection, the patient is placed in the supine position and five laparoscopic ports are placed as with the MIS transhiatal esophagectomy. The abdominal portions of the operation are identical to those described previously in the section entitled “Minimally Invasive Transhiatal Esophagectomy,” and the gastric conduit is then sewn to the tip of the fully mobilized GEJ and lesser curvature sleeve. A feeding tube is placed, and the pyloroplasty may be performed laparoscopically. A transverse cervical incision and dissection between the sternocleidomastoid and the anterior strap muscles allows access to the cervical esophagus. Great care is made to avoid stretching the recurrent laryngeal nerve. The esophagus and proximal stomach is then pulled up into the neck with the gastric conduit following. Cervical anastomosis is then performed. The MIS transthoracic two-field esophagectomy is slightly different. In this operation, the abdominal portions of the operation are done first, including placement of the feeding tube, the creation of the conduit, and the sewing of the tip of the conduit to the fully dissected GEJ. The patient is then rolled into the left lateral decubitus position and, through right thoracoscopy, the esophagus is dissected and divided 10 cm above the tumor. Once freed, the specimen is pulled out through the mini-thoracotomy, and an end-to-end anastomosis stapler is introduced through the high corner of the gastric conduit and out a stab wound along the greater curvature. The anvil of the stapler is placed in the proximal esophagus and held with a pursestring, the stapler is docked, the anastomosis is created, and a gastrotomy is then closed with another firing of the GIA stapler. The three-field esophagectomy has the advantage of placing the anastomosis in the neck where leakage is unlikely to create a severe systemic consequence. On the other hand, placement of the anastomosis in the high chest minimizes the risks of injury to structures in the neck, particularly the recurrent laryngeal nerve. Although the leak of the intrathoracic anastomosis may be more likely to bear septic consequences, the incidence of leak is diminished. Other complications of this approach relate to pulmonary and cardiac status. In many series, the most common complication is pneumonia, the second is atrial fibrillation, and the third is anastomotic leak. Ivor Lewis (En Bloc) Esophagectomy. The theory behind radical transthoracic esophagectomy is that greater removal of LNs and periesophageal tissues diminishes the chance of a positive radial margin and LN recurrence. Although there are no randomized data demonstrating this to be superior to other forms of esophagectomy, there are many retrospective data demonstrating improved survival with greater numbers of LNs harvested. A recent study from Sloan-Kettering demonstrates a direct relationship between the number of negative nodes harvested and long-term survival. Although such a survival advantage may be related to the completeness of resection, extended radical resections may also be a surrogate for experienced surgeons working in great institutions. As a time-honored operation, there is no doubt that en bloc esophagectomy is the standard to which less radical techniques must be compared. Generally, this operation is started in the abdomen with an upper midline laparotomy and extensive LN dissection in and about the celiac access and its branches, extending into the porta hepatis and along the splenic artery to the tail of the pancreas. All LNs are removed en bloc with the lesser curvature of the stomach. Unless the tumor extends into the stomach, reconstruction is performed with a greater curvature gastric tube. For GEJ cancers extending significantly into the gastric cardia or fundus, the proximal stomach is removed, and reconstruction is performed with an isoperistaltic section of left colon between the upper esophagus and the remnant stomach, or the colon is connected to a Roux-en-Y limb of jejunum, if total gastrectomy is necessary. In the majority of cases, colon interposition is unnecessary, and a gastric conduit is used. Following closure of the abdominal incision, the patient is placed in the left lateral decubitus position and an anterolateral thoracotomy is performed through the sixth intercostal space. The azygos vein is divided and the posterior mediastinum is entirely cleaned out to include the thoracic duct, all periaortic tissues, and all tissue in the upper mediastinum along the course of the current laryngeal nerves and in the peribronchial, but when adjusted by cancer stage, this survival benefit disappeared. The mortality and morbidity after transhiatal esophagectomy appeared to be less. Suffice it to say that this debate over the best procedure for esophagectomy remains an open question. The role of the minimally invasive surgical procedures for a cancer cure will require further study and longer follow-up. It would appear from preliminary analysis that the transhiatal esophagectomy, like its open cousin, may be performed with less morbidity and mortality than the VATS procedure. Longterm survival analyses will require careful follow-up for at least 5 to 10 years after cancer treatment. A recent European multicenter randomized trial comparing open and minimally invasive approaches revealed a highly significant reduction in pulmonary complications in the patients who underwent the minimally invasive approach. There was no difference in procedure-related mortality between the approaches. Three-Field Open Esophagectomy. Three-field open esoph- Radiation Therapy. Primary treatment with radiation ther- agectomy is very similar to a minimally invasive three-field except that all access is through open incisions. This procedure is preferred by certain Japanese surgeons and LN counts achieved through this kind of operation may run from 45 to 60 LNs. Most Western surgeons question the benefit of such radical surgery when it is hard to define a survival advantage. Nonetheless, high intrathoracic cancers probably deserve such an aggressive approach if cure is the goal. Salvage Esophagectomy. Salvage esophagectomy is the nomenclature applied to esophagectomy performed after failure of definitive radiation and chemotherapy. The most frequent scenario is one in which distant disease (bone, lung, brain, or wide LN metastases) renders the patient nonoperable at initial presentation. Then, systemic chemotherapy, usually with radiation of the primary tumor, destroys all foci of metastasis, as demonstrated by CT and CT-PET, but the primary remains present and symptomatic. Following a period of observation, to make sure no new disease will become evident, salvage esophagectomy is performed, usually with an open two-field approach. Surprisingly, the cure rate of salvage esophagectomy is not inconsequential. One in four patients undergoing this operation will be disease free 5 years later, despite the presence of residual cancer in the operative specimen. Because of the dense scarring created by radiation treatment, this procedure is the most technically challenging of all esophagectomy techniques. Comparative Studies of Esophagectomy Technique Transthoracic vs. Transhiatal Esophagectomy. There has been a great debate as to whether en bloc esophagectomy will provide a greater long-term benefit and cure rate in esophageal cancer than transhiatal esophagectomy. In a recent 7-year follow-up of a Dutch study addressing GEJ and lower esophageal cancers, there does not appear to be any benefit to the more extensive dissection despite higher morbidity and mortality. In a subgroup analysis of those with one to eight positive LNs, it did appear that the en bloc transthoracic resection may add to longevity. In another large database analysis of the Surveillance, Epidemiology, and End Results database, transthoracic and transhiatal esophagectomy were compared. In this study, the transhiatal esophagectomy had a greater long-term survival, Alternative Therapies apy does not produce results comparable with those obtained with surgery. Currently, the use of radiotherapy is restricted to patients who are not candidates for surgery, and it is usually combined with chemotherapy. Radiation alone is used for palliation of dysphagia, but the benefit is short lived, lasting only 2 to 3 months. Furthermore, the length and course of treatment are difficult to justify in patients with a limited life expectancy. Radiation is effective in patients who have hemorrhage from the primary tumor. Adjuvant Chemotherapy. The proposal to use adjuvant chemotherapy in the treatment of esophageal cancer began when it became evident that most patients develop postoperative systemic metastasis without local recurrence. This observation led to the hypothesis that undetected systemic micrometastasis had been present at the time of diagnosis, and if effective systemic therapy was added to local regional therapy, survival should improve. Recently, this hypothesis has been supported by the observation of epithelial tumor cells in the bone marrow in 37% of patients with esophageal cancer who were resected for cure. These patients had a greater prevalence of relapse at 9 months after surgery compared to those patients without such cells. Such studies emphasize that hematogenous dissemination of viable malignant cells occurs early in the disease, and that systemic chemotherapy may be helpful if the cells are sensitive to the agent. On the other hand, systemic chemotherapy may be a hindrance, because of its immunosuppressive properties, if the cells are resistant. Unfortunately, current technology is not able to test tumor cell sensitivity to chemotherapeutic drugs. This requires that the choice of drugs be made solely on the basis of their clinical effectiveness against grossly similar tumors. The decision to use preoperative rather than postoperative chemotherapy was based on the ineffectiveness of chemotherapeutic agents when used after surgery, and animal studies suggesting that agents given before surgery were more effective. The claim that patients who receive chemotherapy before resection are less likely to develop resistance to the drugs is unsupported by hard evidence. The claim that drug delivery is enhanced because blood flow is more robust before patients undergo surgical dissection is similarly flawed, due to the fact that if enough blood reaches the operative site to heal the wound or anastomosis, then the flow should be sufficient to 1077 CHAPTER 25 ESOPHAGUS AND DIAPHRAGMATIC HERNIA hilar, and tracheal LN stations. The proximal stomach is pulled up into the thorax where a conduit is created (if not performed previously) and a handsewn or stapled anastomosis is made between the upper thoracic esophagus and the gastric conduit or transverse colon. Chest tubes are placed, and the patient is taken to the intensive care unit. Because this is the most radical of dissections, complications are most common, including pneumonia, respiratory failure, atrial fibrillation, chylothorax, anastomotic leak, conduit necrosis, gastrocutaneous fistula, and, if dissection is too near the recurrent laryngeal nerves, hoarseness will occur with an increased risk of aspiration. Tracheobronchial injury resulting in fistulas between the bronchus and conduit may also occur, however rarely. Although this procedure and threefield esophagectomy are fraught with the highest complication rate, the long-term outcome of this procedure provides the greatest survival in many single-center series and retrospective reviews. deliver chemotherapeutic drugs. There are, however, data supporting the claim that preoperative chemotherapy in patients with esophageal carcinoma can, if effective, facilitate surgical resection by reducing the size of the tumor. This is particularly beneficial in the case of squamous cell tumors above the level of the carina. Reducing the size of the tumor may provide a safer margin between the tumor and the trachea and allow an anastomosis to a tumor-free cervical esophagus just below the cricopharyngeus. Involved margin at this level usually requires a laryngectomy to prevent subsequent local recurrence. PART II Preoperative Chemotherapy. Eight randomized prospective studies of neoadjuvant chemotherapy vs. surgery alone have demonstrated mixed results. For adenocarcinomas of the distal esophagus and proximal stomach, preoperative neoadjuvant 5-fluorouracil (5-FU) and cisplatin chemotherapy has been shown to provide a survival advantage over surgery alone in a well-powered study from the United Kingdom (MRC trial). This trial is one of the few to include enough patients (800) to detect small differences. The trial had a 10% absolute survival benefit at 2 years for the neoadjuvant chemotherapy group. In a second trial from the United Kingdom (MAGIC trial) of distal esophageal and proximal gastric adenocarcinomas, the use of epirubicin in combination with cisplatin and 5-FU also demonstrated a survival advantage for the induction chemotherapy arm with 4 years median follow-up. As a result of these two trials, standard treatment of locally advanced adenocarcinoma in Europe calls for neoadjuvant chemotherapy with one of these two regimens. Most failures are due to distant metastatic disease, underscoring the need for improved systemic therapy. Postoperative septic and respiratory complications may be more common in patients receiving chemotherapy. SPECIFIC CONSIDERATIONS 1078 Preoperative Combination Chemo- and Radiotherapy. Preoperative chemoradiotherapy using cisplatin and 5-FU in combination with radiotherapy has been reported by several investigators to be beneficial in both adenocarcinoma and squamous cell carcinoma of the esophagus. There have been 10 randomized prospective studies (Table 25-13). A recent metaanalysis of these trials demonstrates a 13% survival advantage for neoadjuvant chemoradiation therapy, which is more pronounced for patients with adenocarcinoma than for those with squamous carcinoma (Table 25-14). It was also observed that the benefit for chemotherapy alone (7%) was not as dramatic as for chemoradiotherapy used in the neoadjuvant setting. Additionally, other work has demonstrated the importance of obtaining an R0 (tumor-free) resection as the most important variable determining long-term survival. Although there are no direct, randomized comparisons between chemotherapy and chemoradiation therapy, it appears that the addition of radiation may improve local response of the tumor and may allow a greater opportunity for the surgeon to obtain an R0 resection. The timing of surgery after chemoradiation induction is generally felt to be optimal between 6 and 8 weeks following the completion of induction therapy. Earlier than this time, active inflammation may make the resection hazardous, and the patients have not had time to recover fully from the chemoradiation. After 8 weeks, edema in the periesophageal tissue starts to turn to scar tissue, making dissection more difficult. With chemoradiation, the complete response rates for adenocarcinoma range from 17% to 24% (Table 25-15). No tumor is detected in the specimen after esophagectomy. Patients demonstrating a complete response to chemoradiation have a better survival rate than those without complete response, but distant failure remains common. At present, the strongest predictors of outcome of patients with esophageal cancer are the anatomic extent of the tumor at diagnosis and the completeness of tumor removal by surgical resection. After incomplete resection of an esophageal cancer, the 5-year survival rates are 0% to 5%. In contrast, after complete resection, independent of stage of disease, 5-year survival ranges from 15% to 40%, according to selection criteria and stage distribution. The importance of early recognition and adequate surgical resection cannot be overemphasized. Figure 25-70 is a global algorithm for the management of esophageal carcinoma. SARCOMA OF THE ESOPHAGUS Sarcomas and carcinosarcomas are rare neoplasms, accounting for approximately 0.1% to 1.5% of all esophageal tumors. They present with the symptom of dysphagia, which does not differ from the dysphagia associated with the more common epithelial carcinoma. Tumors located within the cervical or high thoracic esophagus can cause symptoms of pulmonary aspiration secondary to esophageal obstruction. Large tumors originating at the level of the tracheal bifurcation can produce symptoms of airway obstruction and syncope by direct compression of the tracheobronchial tree and heart (Fig. 25-71). The duration of dysphagia and age of the patients affected with these tumors are similar to those with carcinoma of the esophagus. A barium swallow usually shows a large polypoid intraluminal esophageal mass, causing partial obstruction and dilatation of the esophagus proximal to the tumor (Fig. 25-72). The smooth polypoid nature of the lesion, although not diagnostic, is distinctive enough to suggest the presence of a sarcoma rather than the more common ulcerating, stenosing carcinoma. Esophagoscopy commonly shows an intraluminal necrotic mass. When biopsy is attempted, it is important to remove the necrotic tissue until bleeding is seen on the tumor’s surface. When this is not done, the biopsy specimen will show only tissue necrosis. Even when viable tumor is obtained on biopsy, it has been these authors’ experience that it cannot be definitively identified as carcinoma, sarcoma, or carcinosarcoma on the basis of the histology of the portion biopsied. Biopsy results cannot be totally relied on to identify the presence of sarcoma, and it is often the polypoid nature of the lesion that arouses suspicion that it may be something other than carcinoma. Polypoid sarcomas of the esophagus, in contrast to infiltrating carcinomas, remain superficial to the muscularis propria and are less likely to metastasize to regional LNs. In one series of 14 patients, local extension or tumor metastasis would have prevented a potentially curative resection in only five. Thus, the presence of a large polypoid tumor should not deter the surgeon from resecting the lesion. Sarcomatous lesions of the esophagus can be divided into epidermoid carcinomas with spindle cell features, such as carcinosarcoma, and true sarcomas that arise from mesenchymal tissue, such as leiomyosarcoma, fibrosarcoma, and rhabdomyosarcoma. Based on current histologic criteria for diagnosis, fibrosarcoma and rhabdomyosarcoma of the esophagus are extremely rare lesions. Surgical resection of polypoid sarcoma of the esophagus is the treatment of choice because radiation therapy has little 1079 Table 25-13 Randomized trials of neoadjuvant chemoradiotherapy vs. surgery, or neoadjuvant chemotherapy vs. surgery YEAR TREATMENT SCHEDULE TREATMENT SCHEDULE ACTIVATED (RADIOTHERAPY) (CHEMOTHERAPY) Two cycles: cisplatin 20 mg/m2 d 1–5; Sequential bleomycin 5 mg/m2 d 1–5 Concurrent Two cycles: cisplatin 100 mg/m2 d 1; 5-fluorouracil 1000 mg/m2 d 1–4 Two cycles: cisplatin 100 mg/m2 d 1; Sequential 5-fluorouracil 600 mg/m2 d 2–5, 22–25 1989 45 Gy, 1.5 Gy/fraction Two cycles: cisplatin 20 mg/m2 d 1–5; Concurrent over 3 wk 5-fluorouracil 300 mg/m2 d 1–21; vinblastine 1 mg/m2 d 1–4 1989 37 Gy, 3.7 Gy/fraction Two cycles: cisplatin 80 mg/m2 d 0–2 Sequential over 2 wk 1990 40 Gy, 2.7 Gy/fraction Two cycles: cisplatin 75 mg/m2 d 7; Concurrent over 3 wk 5-fluorouracil 15 mg/kg d 1–5 1990 40 Gy, 2.7 Gy/fraction Two cycles: cisplatin 75 mg/m2 d 7; Concurrent over 3 wk 5-fluorouracil 15 mg/kg d 1–5 Concurrent 1994 35 Gy, 2.3 Gy/fraction One cycle: cisplatin 80 mg/m2 d 1; over 3 wk 5-fluorouracil 800 mg/m2 d 2–5 2006 50.4 Gy, 1.8 Gy/ Two cycles: cisplatin 60 mg/m2 d 1; Concurrent fraction over 5.6 wk 5-fluorouracil 1000 mg/m2 d 3–5 Concurrent 1999 45.6 Gy, 1.2 Gy/ Two cycles: cisplatin 60 mg/m2 d 1; fraction over 28 d 5-fluorouracil 1000 mg/m2 d 3–5 Chemotherapy 1982 — Two cycles: cisplatin 120 mg/m2 d 1; — vindesine 3 mg/m2 d 1, 8; bleomycin 10 U/m2 d 3–6 1983 — Two cycles: cisplatin 20 mg/m2 d 1–5; — bleomycin 5 mg/m2 d 1–5 c 1988 — Three cycles: cisplatin 20 mg/m2 d 1–5; — 5-fluorouracil 1000 mg/m2 d 1–5 1988 — Two cycles: cisplatin 100 mg/m2 d 1; — bleomycin 10 mg/m2 d 3–8; vinblastine 3 mg/m2 d 1, 8 — 1989 — Two cycles: cisplatin 100 mg/m2 d 1; 5-fluorouracil 1000 mg/m2 d 1–5 1990 — Two cycles: cisplatin 80 mg/m2 d 1; — etoposide 200 mg/m2 d 1–5 — 1990 — Three cycles: cisplatin 100 mg/m2 1; 5-fluorouracil 1000 mg/m2 days 1–5 1992 — Two cycles: cisplatin 100 mg/m2 d 1; — 5-fluorouracil 1000 mg/m2 d 1–5 — 1992 — Two cycles: cisplatin 80 mg/m2 d 1; 5-fluorouracil 1000 mg/m2 d 1–4 MEDIAN SAMPLE FOLLOWSIZE UP (MO) SCC 78 18a SCC 69 12a SCC 86 12a SCC and 100 adenocarcinoma 98 SCC 293 55 Adenocarcinoma 113 24 SCC 61 10 SCC and 256 adenocarcinoma SCC and 56 adenocarcinoma SCC 101 65 60 SCC 39 20 SCC 106 18a SCC 46 75 SCC 46 17a SCC 147 17 SCC 160 19a SCC and 467 adeno-carcinoma SCC 96 56 SCC and 802 adeno-carcinoma 37 25 24 Estimated as median survival. Unpublished thesis. c Year of activation not reported, but imputed. d Only available as an abstract. SCC = squamous cell carcinoma. Reproduced with permission from Gebski V, Burmeister B, Smithers BM, et al: Survival benefits from neoadjuvant chemoradiotherapy or chemotherapy in oesophageal carcinoma: a meta-analysis, Lancet Oncol. 2007 Mar;8(3):226-234. a b CHAPTER 25 ESOPHAGUS AND DIAPHRAGMATIC HERNIA Chemoradiotherapy 1983 35 Gy, 1.75 Gy/ fraction over 4 wk 1986 40 Gy, 2 Gy/fraction over 4 wk 1988 20 Gy, 2 Gy/fraction over 12 d CONCURRENT OR SEQUENTIAL TUMOR TYPE 1080 Table 25-14 Results of the meta-analysis applied to effects of preoperative chemoradiotherapy and chemotherapy on 2-y survival for patients with various levels of risk RISK GROUP 2-Y SURVIVAL RATE (%) EXPECTED 2-Y MORTALITY CONTROL (%) TREATEDa (%) ARR (%) NNT PART II Chemoradiotherapy High 20 80 64.8 15.2 7 Medium 35 65 52.7 12.3 8 Low 50 50 40.5 9.5 10 SPECIFIC CONSIDERATIONS Chemotherapy High 20 80 72.0 12.0 8 Medium 35 65 58.5 6.5 15 Low 50 50 45.0 5.0 20 Based on a 19% relative mortality reduction for those receiving concurrent chemoradiotherapy and a 10% relative mortality reduction for those receiving chemotherapy. ARR = absolute risk reduction; NNT = number needed to treat to prevent one death. Reproduced with permission from Gebski V, Burmeister B, Smithers BM, et al: Survival benefits from neoadjuvant chemoradiotherapy or chemotherapy in oesophageal carcinoma: a meta-analysis, Lancet Oncol. 2007 Mar;8(3):226-234. a success and the tumors remain superficial, with local invasion or distant metastases occurring late in the course of the disease. As with carcinoma, the absence of both wall penetration and LN metastases is necessary for curative treatment, and surgical resection is consequently responsible for the majority of the reported 5-year survivals. Resection also provides an excellent means of palliating the patient’s symptoms. The surgical technique for resection and the subsequent restoration of the GI continuity is similar to that described for carcinoma. In these authors’ experience, four of the eight patients with carcinosarcoma survived for 5 years or longer. Even though this number is small, it suggests that resection produces better results in epithelial carcinoma with spindle cell features than in squamous cell carcinoma of the esophagus. Similarly, with leiomyosarcoma of the esophagus, the same scattered reports exist with little information on survival. Of seven patients with leiomyosarcoma, two died from their disease—one in 3 months and the other 4 years and 7 months after resection. The other five patients were reported to have survived more than 5 years. It is difficult to evaluate the benefits of resection for leiomyoblastoma of the esophagus because of the small number of reported patients with tumors in this location. Most leiomyoblastomas occur in the stomach, and 38% of these patients succumb to the cancer in 3 years. Fifty-five percent of patients with extragastric leiomyoblastoma also die from the disease, within an average of 3 years. Consequently, leiomyoblastoma should be considered a malignant lesion and apt to behave like a leiomyosarcoma. The presence of nuclear hyperchromatism, increased mitotic figures (more than one per high-power field), tumor size larger than 10 cm, and clinical symptoms of longer than 6 months’ duration are associated with a poor prognosis. BENIGN TUMORS AND CYSTS Benign tumors and cysts of the esophagus are relatively uncommon. From the perspectives of both the clinician and the pathologist, benign tumors may be divided into those that are within the muscular wall and those that are within the lumen of the esophagus. Intramural lesions are either solid tumors or cysts, and the vast majority are leiomyomas. They are made up of varying portions of smooth muscle and fibrous tissue. Fibromas, myomas, fibromyomas, and lipomyomas are closely related and occur rarely. Other histologic types of solid intramural tumors have been described, such as lipomas, neurofibromas, hemangiomas, osteochondromas, granular cell myoblastomas, and glomus tumors, but they are medical curiosities. Intraluminal lesions are polypoid or pedunculated growths that usually originate in the submucosa, develop mainly into the lumen, and are covered with normal stratified squamous epithelium. The majority of these tumors are composed of fibrous tissue of varying degrees of compactness with a rich vascular supply. Some are loose and myxoid (e.g., myxoma and myxofibroma), some are more collagenous (e.g., fibroma), and some contain adipose tissue (e.g., fibrolipoma). These different types of tumor are frequently collectively designated fibrovascular Table 25-15 Results of neoadjuvant therapy in adenocarcinoma of the esophagus INSTITUTION YEAR NO. OF PATIENTS REGIMEN COMPLETE PATHOLOGIC RESPONSE (%) SURVIVAL MD Anderson 1990 35 P, E, 5-FU 3 42% at 3 y SLMC 1992 18 P, 5-FU, RT 17 40% at 3 y Vanderbilt 1993 39 P, E, 5-FU, RT 19 47% at 4 y Michigan 1993 21 P, VBL, 5-FU, RT 24 34% at 5 y MGH 1994 16 P, 5-FU 0 42% at 4 y MGH 1994 22 E, A, P 5 58% at 2 y A = doxorubicin; E = etoposide; 5-FU = 5-fluorouracil; MGH = Massachusetts General Hospital; P = cisplatin; RT = radiation therapy; SLMC = St. Louis University Medical Center; VBL = vinblastine. Reproduced with permission from Wright CD, Mathisen DJ, Wain JC, et al: Evolution of treatment strategies for adenocarcinoma of the esophagus and gastroesophageal junction, Ann Thorac Surg. 1994 Dec;58(6):1574-1578. 1081 Barium swallow, endoscopy Clinical evaluation Late disease or significant comorbidity Early disease Advanced disease - Distant organ metastasis - Imminent cardiac pulmonary or hepatic failure Tumor suspected not to be through the wall and/or less than 8 lymph nodes involved Through the wall and multiple lymph node metastasis Chemoradiation Curative en bloc resection Preoperative chemoradiation followed by en bloc resection Treatment failure or recurrence Severe debility Advanced disease Local recurrence No metastases Complete excision possible Unresectable proximal or bleeding tumor Airway fistula or unresectable primary tumor or local recurrence Distant metastasis No local recurrence Supportive care Palliative surgery Laser ablative therapy Stent Chemotherapy Figure 25-70. Suggested global algorithm for the management of carcinoma of the esophagus. CT = computed tomography. polyps, or simply as polyps. Pedunculated intraluminal tumors should be removed. If the lesion is not too large, endoscopic removal with a snare is feasible. Leiomyoma Leiomyomas constitute more than 50% of benign esophageal tumors. The average age at presentation is 38, which is in sharp contrast to that seen with esophageal carcinoma. Leiomyomas are twice as common in males. Because they originate in smooth muscle, 90% are located in the lower two-thirds of the esophagus. They are usually solitary, but multiple tumors have been found on occasion. They vary greatly in size and shape. Actually, tumors as small as 1 cm in diameter and as large as 10 lb have been removed. Typically, leiomyomas are oval. During their growth, they remain intramural, having the bulk of their mass protruding toward the outer wall of the esophagus. The overlying mucosa is freely movable and normal in appearance. Dysphagia and pain are the most common complaints, the two symptoms occurring more frequently together than separately. Bleeding directly related to the tumor is rare, and when hematemesis or melena occur in a patient with an esophageal leiomyoma, other causes should be investigated. A barium swallow is the most useful method to demonstrate a leiomyoma of the esophagus (Fig. 25-73). In profile, the tumor appears as a smooth, semilunar, or crescent-shaped filling defect that moves with swallowing, is sharply demarcated, and is covered and surrounded by normal mucosa. Esophagoscopy should be performed to exclude the reported observation of a coexistence with carcinoma. The freely movable mass, which bulges into the lumen, should not be biopsied because of an increased chance of mucosal perforation at the time of surgical enucleation. Endoscopic ultrasound is also a useful adjunct in the workup of leiomyoma and provides detail related to the anatomic extent and relationship to surrounding structures. Despite their slow growth and limited potential for malignant degeneration, leiomyomas should be removed unless there are specific contraindications. The majority can be removed by simple enucleation. If, during removal, the mucosa is inadvertently entered, the defect can be repaired primarily. After tumor removal, the outer esophageal wall should be reconstructed by closure of the muscle layer. The location of the lesion and the CHAPTER 25 ESOPHAGUS AND DIAPHRAGMATIC HERNIA Tumor staging (CT chest and abdomen, endoscopic ultrasonography) 1082 PART II SPECIFIC CONSIDERATIONS B A Figure 25-71. A. Computed tomographic scan of a leiomyosarcoma (black arrow) that caused compression of the heart and symptoms of syncope. B. Surgical specimen of leiomyosarcoma shown in A with a pedunculated luminal lesion (white arrow) and a large extraesophageal component (black arrow). There was no evidence of lymph node metastasis at the time of operation. A B Figure 25-72. A. Barium swallow showing a large polypoid intraluminal esophageal mass causing partial obstruction and dilation of the proximal esophagus. B. Operative specimen showing 9-cm polypoid leiomyoblastoma. ESOPHAGEAL PERFORATION extent of surgery required will dictate the approach. Lesions of the proximal and middle esophagus require a right thoracotomy, whereas distal esophageal lesions require a left thoracotomy. Videothoracoscopic and laparoscopic approaches are now frequently used. The mortality rate associated with enucleation is low, and success in relieving the dysphagia is near 100%. Large lesions or those involving the GEJ may require esophageal resection. Esophageal Cyst Cysts may be congenital or acquired. Congenital cysts are lined wholly or partly by columnar ciliated epithelium of the respiratory type, by glandular epithelium of the gastric type, by squamous epithelium, or by transitional epithelium. In some, epithelial lining cells may be absent. Confusion over the embryologic origin of congenital cysts has led to a variety of names, such as enteric, bronchogenic, duplication, and mediastinal cysts. Acquired retention cysts also occur, probably as a result of obstruction of the excretory ducts of the esophageal glands. Enteric and bronchogenic cysts are the most common, and they arise as a result of developmental abnormalities during the formation and differentiation of the lower respiratory tract, esophagus, and stomach from the foregut. During its embryologic development, the esophagus is lined successively with simple columnar, pseudostratified ciliated columnar, and, finally, stratified squamous epithelium. This sequence probably accounts for the fact that the lining epithelium may be any or a combination of these; the presence of cilia does not necessarily indicate a respiratory origin. Cysts vary in size from small to very large, and they are usually located intramurally in the middle- to lower-third of the esophagus. Their symptoms are similar to those of a leiomyoma. The diagnosis similarly depends on radiographic, endoscopic, and endosonographic findings. Surgical excision by enucleation is the preferred treatment. During removal, a fistulous tract connecting the cysts to the airways should be sought, particularly in patients who have had repetitive bronchopulmonary infections. Diagnosis Abnormalities on the chest radiogram can be variable and should not be depended upon to make the diagnosis. This is because the abnormalities are dependent on three factors: (a) the time interval between the perforation and the radiographic examination, (b) the site of perforation, and (c) the integrity of the mediastinal pleura. Mediastinal emphysema, a strong indicator of perforation, takes at least 1 hour to be demonstrated and is present in only 40% of patients. Mediastinal widening secondary to edema may not occur for several hours. The site of perforation also can influence the radiographic findings. In cervical perforation, cervical emphysema is common and mediastinal emphysema rare; the converse is true for thoracic perforations. CHAPTER 25 ESOPHAGUS AND DIAPHRAGMATIC HERNIA Figure 25-73. Barium esophagogram showing a classical, smooth, contoured, punched-out defect of a leiomyoma. Perforation of the esophagus constitutes a true emergency. It most commonly occurs following diagnostic or therapeutic procedures. Spontaneous perforation, referred to as Boerhaave’s syndrome, accounts for only 15% of cases of esophageal perforation, foreign bodies for 14%, and trauma for 10%. Pain is a striking and consistent symptom and strongly suggests that an esophageal rupture has occurred, particularly if located in the cervical area following instrumentation of the esophagus, or substernally in a patient with a history of resisting vomiting. If subcutaneous emphysema is present, the diagnosis is almost certain. Spontaneous rupture of the esophagus is associated with a high mortality rate because of the delay in recognition and treatment. Although there usually is a history of resisting vomiting, in a small number of patients, the injury occurs silently, without any antecedent history. When the chest radiogram of a patient with an esophageal perforation shows air or an effusion in the pleural space, the condition is often misdiagnosed as a pneumothorax or pancreatitis. An elevated pleural amylase caused by the extrusion of saliva through the perforation may fix the diagnosis of pancreatitis in the mind of an unwary physician. If the chest radiogram is normal, a mistaken diagnosis of myocardial infarction or dissecting aneurysm is often made. Spontaneous rupture usually occurs into the left pleural cavity or just above the GEJ. About 50% of patients have concomitant GERD, suggesting that minimal resistance to the transmission of abdominal pressure into the thoracic esophagus is a factor in the pathophysiology of the lesion. During vomiting, high peaks of intragastric pressure can be recorded, frequently exceeding 200 mmHg, but because extragastric pressure remains almost equal to intragastric pressure, stretching of the gastric wall is minimal. The amount of pressure transmitted to the esophagus varies considerably, depending on the position of the GEJ. When it is in the abdomen and exposed to intraabdominal pressure, the pressure transmitted to the esophagus is much less than when it is exposed to the negative thoracic pressure. In the latter situation, the pressure in the lower esophagus will frequently equal intragastric pressure if the glottis remains closed. Cadaver studies have shown that when this pressure exceeds 150 mmHg, rupture of the esophagus is apt to occur. When a hiatal hernia is present and the sphincter remains exposed to abdominal pressure, the lesion produced is usually a Mallory-Weiss mucosal tear, and bleeding rather than perforation is the problem. This is due to the stretching of the supradiaphragmatic portion of the gastric wall. In this situation, the hernia sac represents an extension of the abdominal cavity, and the GEJ remains exposed to abdominal pressure. 1083 1084 PART II SPECIFIC CONSIDERATIONS Figure 25-75. Radiographic study of a patient with a perforation of the esophagus using water-soluble contrast material. The patient is placed in the lateral decubitus position with the left side up to allow complete filling of the esophagus and demonstration of the defect. Figure 25-74. Chest radiogram showing air in the deep muscles of the neck following perforation of the esophagus (arrow). This is often the earliest sign of perforation and can be present without evidence of air in the mediastinum. Frequently, air will be visible in the erector spinae muscles on a neck radiogram before it can be palpated or seen on a chest radiogram (Fig. 25-74). The integrity of the mediastinal pleura influences the radiographic abnormality in that rupture of the pleura results in a pneumothorax, a finding that is seen in 77% of patients. In two-thirds of patients, the perforation is on the left side; in one-fifth, it is on the right side; and in one-tenth, it is bilateral. If pleural integrity is maintained, mediastinal emphysema (rather than a pneumothorax) appears rapidly. A pleural effusion secondary to inflammation of the mediastinum occurs late. In 9% of patients, the chest radiogram is normal. The diagnosis is confirmed with a contrast esophagram, which will demonstrate extravasation in 90% of patients. The use of a water-soluble medium such as Gastrografin is preferred. Of concern is that there is a 10% false-negative rate. This may be due to obtaining the radiographic study with the patient in the upright position. When the patient is upright, the passage of water-soluble contrast material can be too rapid to demonstrate a small perforation. The studies should be done with the patient in the right lateral decubitus position (Fig. 25-75). In this, the contrast material fills the entire length of the esophagus, allowing the actual site of perforation and its interconnecting cavities to be visualized in almost all patients. To get adequate exposure of the injury, a dissection similar to that described for esophageal myotomy is performed. A flap of stomach is pulled up and the soiled fat pad at the GEJ is removed. The edges of the injury are trimmed and closed primarily (Fig. 25-77). The closure is reinforced with the use of a pleural patch or construction of a Nissen fundoplication. Mortality associated with immediate closure varies between 8% and 20%. After 24 hours, survival decreases to <50%, and is not influenced by the type of operative therapy (i.e., drainage alone or drainage plus closure of the perforation). If the time delay before closing a perforation approaches 24 hours and the tissues are inflamed, division of the cardia and resection of the diseased portion of the esophagus are recommended. The remainder of the esophagus is mobilized, and as much normal esophagus as possible is saved and brought out as an end cervical esophagostomy. In some situations, the retained esophagus may be so long that Management The key to optimum management is early diagnosis. The most favorable outcome is obtained following primary closure of the perforation within 24 hours, resulting in 80% to 90% survival. Figure 25-76 is an operative photograph taken through a left thoracotomy of an esophageal rupture following a pneumatic dilation for achalasia. The most common location for the injury is the left lateral wall of the esophagus, just above the GEJ. Figure 25-76. Left thoracotomy in a patient with an esophageal rupture at the gastroesophageal junction following forceful dilation of the lower esophagus for achalasia (the surgical clamp is on the stomach, and the Penrose drain encircles the esophagus). The injury consists of a mucosal perforation and extensive splitting of the esophageal muscle from just below the Penrose drain to the stomach. 1085 conditions are met, it is reasonable to treat the patient with hyperalimentation, antibiotics, and cimetidine to decrease acid secretion and diminish pepsin activity. Oral intake is resumed in 7 to 14 days, dependent on subsequent radiographic examinations. MALLORY-WEISS SYNDROME Figure 25-77. The technique of closure of an esophageal perforation through a left thoracotomy. A. A tongue of stomach is pulled up through the esophageal hiatus, and the gastroesophageal fat pad is removed; the edges of the mucosal injury are trimmed and closed using interrupted modified Gambee stitches. B. Reinforcement of the closure with a parietal pleural patch. it loops down into the chest. The contaminated mediastinum is drained and a feeding jejunostomy tube is inserted. The recovery from sepsis is often immediate, dramatic, and reflected by a marked improvement in the patient’s condition over a 24-hour period. On recovery from the sepsis, the patient is discharged and returns on a subsequent date for reconstruction with a substernal colon interposition. Failure to apply this aggressive therapy can result in a mortality rate in excess of 50% in patients in whom the diagnosis has been delayed. Nonoperative management of esophageal perforation has been advocated in select situations. The choice of conservative therapy requires skillful judgment and necessitates careful radiographic examination of the esophagus. This course of management usually follows an injury occurring during dilation of esophageal strictures or pneumatic dilations of achalasia. Conservative management should not be used in patients who have free perforations into the pleural space. Cameron proposed three criteria for the nonoperative management of esophageal perforation: (a) the esophagram must show the perforation to be contained within the mediastinum and drain well back into the esophagus (Fig. 25-78), (b) symptoms should be mild, and (c) there should be minimal evidence of clinical sepsis. If these In 1929, Mallory and Weiss described four patients with acute upper GI bleeding who were found at autopsy to have mucosal tears at the GEJ. This syndrome, characterized by acute upper GI bleeding following vomiting, is considered to be the cause of up to 15% of all severe upper GI bleeds. The mechanism is similar to spontaneous esophageal perforation: an acute increase in intra-abdominal pressure against a closed glottis in a patient with a hiatal hernia. Mallory-Weiss tears are characterized by arterial bleeding, which may be massive. Vomiting is not an obligatory factor, as there may be other causes of an acute increase in intraabdominal pressure, such as paroxysmal coughing, seizures, and retching. The diagnosis requires a high index of suspicion, particularly in the patient who develops upper GI bleeding following prolonged vomiting or retching. Upper endoscopy confirms the suspicion by identifying one or more longitudinal fissures in the mucosa of the herniated stomach as the source of bleeding. In the majority of patients, the bleeding will stop spontaneously with nonoperative management. In addition to blood replacement, the stomach should be decompressed and antiemetics administered, as a distended stomach and continued vomiting aggravate further bleeding. A Sengstaken-Blakemore tube will not stop the bleeding, as the pressure in the balloon is not sufficient to overcome arterial pressure. Endoscopic injection of epinephrine may be therapeutic if bleeding does not stop spontaneously. Only occasionally will surgery be required to stop blood loss. The procedure consists of laparotomy and high gastrotomy with oversewing of the linear tear. Mortality is uncommon, and recurrence is rare. CHAPTER 25 ESOPHAGUS AND DIAPHRAGMATIC HERNIA Figure 25-78. Barium esophagogram showing a stricture and a contained perforation following dilation. The injury meets Cameron criteria: It is contained within the mediastinum and drawn back into the esophagus, the patient had mild symptoms, and there was no evidence of clinical sepsis. Nonoperative management was successful. 1086 CAUSTIC INJURY Accidental caustic lesions occur mainly in children, and, in general, rather small quantities of caustics are taken. In adults or teenagers, the swallowing of caustic liquids is usually deliberate, during a suicide attempt, and greater quantities are swallowed. Alkalis are more frequently swallowed accidentally than acids, because strong acids cause an immediate burning pain in the mouth. Pathology PART II SPECIFIC CONSIDERATIONS The swallowing of caustic substances causes an acute and a chronic injury. During the acute phase, care focuses on controlling the immediate tissue injury and the potential for perforation. During the chronic phase, the focus is on treatment of strictures and disturbances in pharyngeal swallowing. In the acute phase, the degree and extent of the lesion are dependent on several factors: the nature of the caustic substance, its concentration, the quantity swallowed, and the time the substance is in contact with the tissues. Acids and alkalis affect tissue in different ways. Alkalis dissolve tissue, and therefore penetrate more deeply, while acids cause a coagulative necrosis that limits their penetration. Animal experiments have shown that there is a correlation between the depth of the lesion and the concentration of sodium hydroxide solution. When a solution of 3.8% comes into contact with the esophagus for 10 seconds, it causes necrosis of the mucosa and the submucosa but spares the muscular layer. A concentration of 22.5% penetrates the whole esophageal wall and into the periesophageal tissues. Cleansing products can contain up to 90% sodium hydroxide. The strength of esophageal contractions varies according to the level of the esophagus, being weakest at the striated muscle–smooth muscle interface. Consequently, clearance from this area may be somewhat slower, allowing caustic substances to remain in contact with the mucosa longer. This explains why the esophagus is preferentially and more severely affected at this level than in the lower portions. The lesions caused by lye injury occur in three phases. First is the acute necrotic phase, lasting 1 to 4 days after injury. During this period, coagulation of intracellular proteins results in cell necrosis, and the living tissue surrounding the area of necrosis develops an intense inflammatory reaction. Second is the ulceration and granulation phase, starting 3 to 5 days after injury. During this period, the superficial necrotic tissue sloughs, leaving an ulcerated, acutely inflamed base, and granulation tissue fills the defect left by the sloughed mucosa. This phase lasts 10 to 12 days, and it is during this period that the esophagus is the weakest. Third is the phase of cicatrization and scarring, which begins the third week following injury. During this period, the previously formed connective tissue begins to contract, resulting in narrowing of the esophagus. Adhesions between granulating areas occur, resulting in pockets and bands. It is during this period that efforts must be made to reduce stricture formation. Clinical Manifestations The clinical picture of an esophageal burn is determined by the degree and extent of the lesion. In the initial phase, complaints consist of pain in the mouth and substernal region, hypersalivation, pain on swallowing, and dysphagia. The presence of fever is strongly correlated with the presence of an esophageal lesion. Bleeding can occur, and, frequently, the patient vomits. These initial complaints disappear during the quiescent period of ulceration and granulation. During the cicatrization and scarring phase, the complaint of dysphagia reappears and is due to fibrosis and retraction, resulting in narrowing of the Table 25-16 Endoscopic grading of corrosive esophageal and gastric burns First degree: Mucosal hyperemia and edema Second degree: Limited hemorrhage, exudate ulceration, and pseudomembrane formation Third degree: Sloughing of mucosa, deep ulcers, massive hemorrhage, complete obstruction of lumen by edema, charring, and perforation esophagus. Of the patients who develop strictures, 60% do so within 1 month, and 80% within 2 months. If dysphagia does not develop within 8 months, it is unlikely that a stricture will occur. Serious systemic reactions such as hypovolemia and acidosis resulting in renal damage can occur in cases in which the burns have been caused by strong acids. Respiratory complications such as laryngospasm, laryngoedema, and occasionally pulmonary edema can occur, especially when strong acids are aspirated. Inspection of the oral cavity and pharynx can indicate that caustic substances were swallowed, but does not reveal that the esophagus has been burned. Conversely, esophageal burns can be present without apparent oral injuries. Because of this poor correlation, early esophagoscopy is advocated to establish the presence of an esophageal injury. To lessen the chance of perforation, the scope should not be introduced beyond the proximal esophageal lesion. The degree of injury can be graded according to the criteria listed in Table 25-16. Even if the esophagoscopy is normal, strictures may appear later. Radiographic examination is not a reliable means to identify the presence of early esophageal injury, but it is important in later follow-up to identify strictures. The most common locations of caustic injuries are shown in Table 25-17. Treatment Treatment of a caustic lesion of the esophagus is directed toward management of both the immediate and late consequences of the injury. The immediate treatment consists of limiting the burn by administering neutralizing agents. To be effective, this must be done within the first hour. Lye or other alkali can be neutralized with half-strength vinegar, lemon juice, or orange juice. Acid can be neutralized with milk, egg white, or antacids. Sodium bicarbonate is not used because it generates carbon dioxide, Table 25-17 Location of caustic injury (n = 62) Pharynx 10% Esophagus 70% Upper 15% Middle 65% Lower 2% Whole 18% Stomach 20% Antral 91% Whole 9% Both stomach and esophagus 14% 1087 Ingestion of caustic agent Esophagoscopy (Within 12 hours) 1° burn 2° & 3° burn Observation 24–48 hours Exploratory laparotomy Viable esophagus and stomach Questionable esophagus and stomach Full thickness necrosis of esophagus and stomach - Intraluminal esophageal stent - Second look at 36 hours - Esophagogastric resection - Posterior gastric wall biopsy - Jejunostomy - Cervical esophagostomy - Jejunostomy - Resection of adjacent involved organs Figure 25-79. Algorithm summarizing the management of acute caustic injury. Figure 25-80. The use of an esophageal stent to prevent stricture. The stent is constructed from a chest tube and placed in the esophagus at the time of an exploratory laparotomy. A Penrose drain is placed over the distal end as a flap valve to prevent reflux. The stent is supported at its upper end by attaching it to a suction catheter that is secured to the nares. Continuous suction removes saliva and mucus trapped in the pharynx and upper esophagus. CHAPTER 25 ESOPHAGUS AND DIAPHRAGMATIC HERNIA which might increase the danger of perforation. Emetics are contraindicated because vomiting renews the contact of the caustic substance with the esophagus and can contribute to perforation if too forceful. Hypovolemia is corrected, and broad-spectrum antibiotics are administered to lessen the inflammatory reaction and prevent infectious complications. If necessary, a feeding jejunostomy tube is inserted to provide nutrition. Oral feeding can be started when the dysphagia of the initial phase has regressed. In the past, surgeons waited until the appearance of a stricture before starting treatment. Currently, dilations are started the first day after the injury, with the aim of preserving the esophageal lumen by removing the adhesions that occurred in the injured segments. However, this approach is controversial in that dilations can traumatize the esophagus, causing bleeding, and perforation, and there are data indicating that excessive dilations cause increased fibrosis secondary to the added trauma. The use of steroids to limit fibrosis has been shown to be effective in animals, but their effectiveness in human beings has not been established. Extensive necrosis of the esophagus frequently leads to perforation, and it is best managed by resection. When there is extensive gastric involvement, the esophagus is nearly always necrotic or severely burned, and total gastrectomy and near-total esophagectomy are necessary. The presence of air in the esophageal wall is a sign of muscle necrosis and impending perforation and is a strong indication for esophagectomy. Management of acute injury is summarized in the algorithm in Fig. 25-79. Some authors have advocated the use of an intraluminal esophageal stent (Fig. 25-80) in patients who are operated on and found to have no evidence of extensive esophagogastric necrosis. In these patients, a biopsy of the posterior gastric wall should be performed to exclude occult injury. If, histologically, there is a question of viability, a second-look operation should be done within 36 hours. If a stent is inserted, it should be kept in position for 21 days, and removed after a satisfactory barium esophagogram. Esophagoscopy should be done, and if strictures are present, dilations initiated. Once the acute phase has passed, attention is turned to the prevention and management of strictures. Both antegrade dilation with a Hurst or Maloney bougie and retrograde dilation with a Tucker bougie have been satisfactory. In a series of 1079 patients, early dilations started during the acute phase gave excellent results in 78%, good results in 13%, and poor results in 2%. During the treatment, 55 patients died. In contrast, of 333 patients whose strictures were dilated when they became symptomatic, only 21% had excellent results, 46% good, and 6% poor, with three dying during the process. The length of time the surgeon should persist with dilation before consideration of esophageal resection is problematic. An adequate lumen should be re-established within 6 months to 1 year, with progressively longer intervals between dilations. If, during the course of treatment, an adequate lumen cannot be established or maintained (i.e., smaller bougies must be used), operative intervention should be considered. Surgical intervention is indicated when there is (a) complete stenosis in which all attempts from above and below have failed to establish a lumen, (b) marked irregularity and pocketing on barium swallow, (c) the development of a severe periesophageal reaction or mediastinitis with dilatation, (d) a fistula, (e) the inability to dilate or maintain the lumen above a 40F bougie, or (f) a patient who is unwilling or unable to undergo prolonged periods of dilation. 1088 PART II SPECIFIC CONSIDERATIONS The variety of abnormalities seen requires that creativity be used when considering esophageal reconstruction. Skin tube esophagoplasties are now used much less frequently than they were in the past, and are mainly of historical interest. Currently, the stomach, jejunum, and colon are the organs used to replace the esophagus, through either the posterior mediastinum or the retrosternal route. A retrosternal route is chosen when there has been a previous esophagectomy or there is extensive fibrosis in the posterior mediastinum. When all factors are considered, the order of preference for an esophageal substitute is (a) colon, (b) stomach, and (c) jejunum. Free jejunal grafts based on the superior thyroid artery have provided excellent results. Whatever method is selected, it must be emphasized that these procedures cannot be taken lightly; minor errors of judgment or technique may lead to serious or even fatal complications. Critical in the planning of the operation is the selection of cervical esophagus, pyriform sinus, or posterior pharynx as the site for proximal anastomosis. The site of the upper anastomosis depends on the extent of the pharyngeal and cervical esophageal damage encountered. When the cervical esophagus is destroyed and a pyriform sinus remains open the anastomosis can be made to the hypopharynx (Fig. 25-81). When the pyriform sinuses are completely stenosed, a transglottic approach is used to perform an anastomosis to the posterior oropharyngeal wall (Fig. 25-82). This allows excision of supraglottic strictures and elevation and anterior tilting of the larynx. In both of these situations, the patient must relearn to swallow. Recovery is long and difficult and may require several endoscopic dilations—and often reoperations. Sleeve resections of short strictures are not successful because the extent of damage to the wall of the esophagus can be greater than realized, and almost invariably the anastomosis is carried out in a diseased area. The management of a bypassed damaged esophagus after injury is problematic. If the esophagus is left in place, ulceration from gastroesophageal reflux or the development of carcinoma must be considered. The extensive dissection necessary to remove the esophagus, particularly in the presence of marked periesophagitis, is associated with significant morbidity. Leaving the esophagus in place preserves the function of the Figure 25-81. Anastomosis of the bowel to a preserved pyriform sinus. To identify the site, a finger is inserted into the free pyriform sinus through a suprahyoid incision (dotted line). This requires removing the lateral inferior portion of the thyroid cartilage as shown in cross-section. Figure 25-82. Anastomosis of the bowel to the posterior oropharynx. The anastomosis is done through an inverted trapezoid incision above the thyroid cartilage (dotted line). A triangle-shaped piece of the upper half of the cartilage is resected. Closure of the oropharynx is done so that the larynx is pulled up (sagittal section). vagus nerves, and, in turn, the function of the stomach. On the other hand, leaving a damaged esophagus in place can result in multiple blind sacs and subsequent development of mediastinal abscesses years later. Most experienced surgeons recommend that the esophagus be removed unless the operative risk is unduly high. ACQUIRED FISTULA The esophagus lies in close contact with the membranous portion of the trachea and left bronchus, predisposing to the formation of fistula to these structures. Most acquired esophageal fistulas are to the tracheobronchial tree and secondary to either esophageal or pulmonary malignancy. Traumatic fistulas and those associated with esophageal diverticula account for the remainder. Fistulas associated with traction diverticula are usually due to mediastinal inflammatory disease, and traumatic fistulas usually occur secondary to penetrating wounds, lye ingestion, or iatrogenic injury. These fistulas are characterized by paroxysmal coughing following the ingestion of liquids, and by recurrent or chronic pulmonary infections. The onset of cough immediately after swallowing suggests aspiration, whereas a brief delay (30–60 seconds) suggests a fistula. Spontaneous closure is rare, owing to the presence of malignancy or a recurrent infectious process. Surgical treatment of benign fistulas consists of division of the fistulous tract, resection of irreversibly damaged lung tissue, and closure of the esophageal defect. To prevent recurrence, a pleural flap should be interposed. Treatment of malignant fistulas is difficult, particularly in the presence of prior irradiation. Generally, only palliative treatment is indicated. This can best be done by using a specially designed esophageal endoprosthesis that bridges and occludes the fistula, allowing the patient to eat. A salivary tube is also a good option for proximal esophageal fistulas. This tube has a proximal “lip” that rests on the cricopharyngeal muscle and thereby directs the saliva into the tube and past the fistula. Rarely, esophageal diversion, coupled with placement of a feeding jejunostomy, can be used as a last resort. TECHNIQUES OF ESOPHAGEAL RECONSTRUCTION Partial Esophageal Resection Distal benign lesions, with preserved proximal esophageal function, are best treated with the interposition of a segment of proximal jejunum into the chest and primary anastomosis. A jejunal interposition can reach to the inferior border of the pulmonary hilum with ease, but the architecture of its blood supply rarely allows the use of the jejunum proximal to this point. Because the anastomosis is within the chest, a thoracotomy is necessary. The jejunum is a dynamic graft and contributes to bolus transport, whereas the stomach and colon function more as a conduit. The stomach is a poor choice in this circumstance because of the propensity for the reflux of gastric contents into the proximal remaining esophagus following an intrathoracic esophagogastrostomy. It is now well recognized that this occurs and can lead to incapacitating symptoms and esophageal destruction in some patients. Short segments of colon, on the other hand, lack significant motility and have a propensity for the development of esophagitis proximal to the anastomosis. Replacement of the cervical portion of the esophagus, while preserving the distal portion, is occasionally indicated in cervical esophageal or head and neck malignancy, and following the ingestion of lye. Free transfer of a portion of jejunum to the neck has become a viable option and is successful in the majority of cases. Revascularization is achieved via use 1089 Reconstruction After Total Esophagectomy Neither the intrathoracic stomach nor the intrathoracic colon functions as well as the native esophagus after an esophagogastrectomy. The choice between these organs will be influenced by several factors, such as the adequacy of their blood supply and the length of resected esophagus that they are capable of bridging. If the stomach shows evidence of disease, or has been contracted or reduced by previous gastric surgery, the length available for esophageal replacement may not be adequate. The presence of diverticular disease, unrecognized carcinoma, or colitis prohibits the use of the colon. The blood supply of the colon is more affected by vascular disease than the blood supply of the stomach, which may prevent its use. Of the two, the colon provides the longest graft. The stomach can usually reach to the neck if the amount of lesser curvature resected does not interfere with the blood supply to the fundus. Gastric interposition has the advantage that only one anastomosis is required. On the other hand, there is greater potential for aspiration of gastric juice or stricture of the cervical anastomosis from chronic reflux when stomach is used for replacement. Following an esophagogastrectomy, patients may have discomfort during or shortly after eating. The most common symptom is a postprandial pressure sensation or a feeling of being full, which probably results from the loss of the gastric reservoir. This symptom is less common when the colon is used as an esophageal substitute, probably because the distal third of the stomach is retained in the abdomen and the interposed colon provides an additional reservoir function. King and Hölscher have reported a 40% and 50% incidence of dysphagia after reestablishing GI continuity with the stomach following esophagogastrectomy. This incidence is similar to Orringer’s results after using the stomach to replace the esophagus in patients with benign disease. More than onehalf of the patients experienced dysphagia postoperatively; Figure 25-83. A. The portion of the thoracic inlet to be resected to provide space for a free jejunal graft and access to the internal mammary artery (shaded area). B. Cross-section showing the space available after resection of the sternoclavicular joint and one-half of the manubrium. (Reproduced with permission from Shields TW: General Thoracic Surgery, 3rd ed. Philadelphia, PA: Lea & Febiger; 1989.) CHAPTER 25 ESOPHAGUS AND DIAPHRAGMATIC HERNIA Options for esophageal substitution include gastric advancement, colonic interposition, and either jejunal free transfer or advancement into the chest. Rarely, combinations of these grafts will be the only possible option. The indications for esophageal resection and substitution include malignant and end-stage benign disease. The latter includes reflux- or drug-induced stricture formation that cannot be dilated without damage to the esophagus, a dilated and tortuous esophagus secondary to severe motility disorders, lye-induced strictures, and multiple previous antireflux procedures. The choice of esophageal substitution has significant impact upon the technical difficulty of the procedure and influences the long-term outcome. of the internal mammary artery and the internal mammary or innominate vein. Removal of the sternoclavicular joint aids in performing the vascular and distal esophageal anastomosis (Fig. 25-83). 1090 PART II SPECIFIC CONSIDERATIONS two-thirds of this group required postoperative dilation, and one-fourth had persistent dysphagia and required home dilation. In contrast, dysphagia is uncommon, and the need for dilation is rare following a colonic interposition. Isolauri reported on 248 patients with colonic interpositions and noted a 24% incidence of dysphagia 12 months after the operation. When it occurred, the most common cause was recurrent mediastinal tumor. The high incidence of dysphagia with the use of the stomach is probably related to the esophagogastric anastomosis in the neck and the resulting difficulty of passing a swallowed bolus. Another consequence of the transposition of the stomach into the chest is the development of postoperative duodenogastric reflux, probably due to pyloric denervation, and adding a pyloroplasty may worsen this problem. Following gastric advancement, the pylorus lies at the level of the esophageal hiatus, and a distinct pressure differential develops between the intrathoracic gastric and intra-abdominal duodenal lumina. Unless the pyloric valve is extremely efficient, the pressure differential will encourage reflux of duodenal contents into the stomach. Duodenogastric reflux is less likely to occur following colonic interposition because there is sufficient intraabdominal colon to be compressed by the abdominal pressure and the pylorus and duodenum remain in their normal intraabdominal position. Although there is general acceptance of the concept that an esophagogastric anastomosis in the neck results in less postoperative esophagitis and stricture than one at a lower level, reflux esophagitis following a cervical anastomosis does occur, albeit at a lower rate than when the anastomosis is at a lower level. Most patients undergo cervical esophagogastrostomy for malignancy; thus, the long-term sequelae of an esophagogastric anastomosis in the neck are not of concern. However, patients who have had a cervical esophagogastrostomy for benign disease may develop problems associated with the anastomosis in the fourth or fifth postoperative year that are severe enough to require anastomotic revision. This is less likely in patients who have had a colonic interposition for esophageal replacement. Consequently, in patients who have a benign process or a potentially curable carcinoma of the esophagus or cardia, a colonic interposition is used to obviate the late problems associated with a cervical esophagogastrostomy. Colonic interposition for esophageal substitution is a more complex procedure than gastric advancement, with the potential for greater perioperative morbidity, particularly in inexperienced hands. Composite Reconstruction Occasionally, a combination of colon, jejunum, and stomach is the only reconstructive option available. This situation may arise when there has been previous gastric or colonic resection, when dysphagia has recurred after a previous esophageal resection, or following postoperative complications such as ischemia of an esophageal substitute. Although not ideal, combinations of colon, jejunum, and stomach used to restore GI continuity function surprisingly well and allow alimentary reconstruction in an otherwise impossible situation. Vagal Sparing Esophagectomy With Colon Interposition Traditional esophagectomy typically results in bilateral vagotomy and its attendant consequences. It is likely that symptoms such as dumping, diarrhea, early satiety, and weight loss seen in 15% to 20% of patients postesophagectomy are at least in part, if not completely, due to vagal interruption. The technique of vagal sparing esophagectomy with colon interposition has been described in an effort to avoid the morbidities associated with standard esophagectomy. Through an upper midline abdominal incision, the right and left vagal nerves are identified, circled with a tape, and retracted to the right. A limited, highly selective proximal gastric vagotomy is performed along the cephalad 4 cm of the lesser curvature. The stomach is divided with an Endo-GIA stapler just below the GEJ. The colon is prepared to provide an interposed segment as previously described. A neck incision is made along the anterior border of the left sternocleidomastoid muscle, and the strap muscles are exposed. The omohyoid muscle is divided at its pulley, and the sternohyoid and sternothyroid muscles are divided at their manubrial insertion. The left carotid sheath is retracted laterally and the thyroid and trachea medially. The left inferior thyroid artery is ligated laterally as it passes under the left common carotid artery. The left recurrent laryngeal nerve is identified and protected. The esophagus is dissected circumferentially in an inferior direction, from the left neck to the apex of the right chest, to avoid injury to the right recurrent laryngeal nerve. The esophagus is divided at the level of the thoracic inlet, leaving about 3 to 4 cm of cervical esophagus. The proximal esophagus is retracted anteriorly and to the right with the use of two sutures to keep saliva and oral contents from contaminating the neck wound. Returning to the abdomen, the proximal staple line of the gastric division is opened, and the esophagus is flushed with povidone-iodine solution. A vein stripper is passed up the esophagus into the neck wound. The distal portion of the esophagus in the neck is secured tightly around the stripping cable with “endoloops” and an umbilical tape for a trailer. The tip of the stripper is exchanged for a mushroom head, and the stripper is pulled back into the abdomen, inverting the esophagus as it transverses the posterior mediastinum. This maneuver strips the branches of the esophageal plexus off the longitudinal muscle of the esophagus, preserving the esophageal plexus along with the proximal vagal nerves and the distal vagal nerve trunks. In patients with end-stage achalasia, only the mucosa is secured around the stripping cable, so that it alone is stripped and the dilated muscular wall of the esophagus, with its enriched blood supply, remains. The resulting mediastinal tunnel, or in the case of achalasia the muscular tube, is dilated with a Foley catheter containing 90 mL of fluid in the balloon. The previously prepared interposed portion of the transverse colon is passed behind the stomach and up through the mediastinal tunnel into the neck. An end-to-end anastomosis is performed to the cervical esophagus using a single layer technique. The colon is pulled taut and secured to the left crus with four or five interrupted sutures. Five centimeters below the crura an opening is made in the mesentery adjacent to the colon along its mesenteric border, through which an EndoGIA stapler is passed and the colon is divided. The proximal end, which is the distal end of the interposed colon, is anastomosed high on the posterior fundic wall of the stomach, using a triangular stapling anastomotic technique. This is done by stapling longitudinally the stomach and colon together with a 75-mm Endo-GIA stapler, spreading the base of the incision apart, and closing it with a T-55 stapler. Colonic continuity is reestablished by bringing the proximal right colon to the distal staple line in the left colon and performing an end-to-end anastomosis using a double-layer technique. BIBLIOGRAPHY Entries highlighted in bright blue are key references. General References Balaji B, Peters JH. Minimally invasive surgery for esophageal motor disorders. Surg Clin North Am. 2002;82:763-782. Bremner CG, DeMeester TR, Bremner RM. Esophageal Motility Testing Made Easy. St. Louis: Quality Medical Publishing, 2001. Castel DW, Richter J, eds. The Esophagus. Boston: Little, Brown & Co., 1999. DeMeester SR, Peters JH, DeMeester TR. Barrett’s esophagus. Curr Probl Surg. 2001;38:549-640. Demeester SR, ed. Barrett’s esophagus. Problems in General Surgery. Vol. 18, no. 2. Hagerstown, MD: Lippincott Williams & Wilkins; 2001. DeMeester TR, Peters JH, Bremner CG, et al. Biology of gastroesophageal reflux disease; pathophysiology relating to medical and surgical treatment. Annu Rev Med. 1999;50:469-506. Hunter JG, Pellagrini CA. Surgery of the esophagus. Surg Clin North Am. 1997;77:959-970. McFadyen BV, Arregui ME, Eubanks S, et al. Laparoscopic Surgery of the Abdomen. New York: Springer, 2003. Surgical Anatomy Daffner RH, Halber MD, Postlethwait RW, et al. CT of the esophagus. II. Carcinoma. AJR Am J Roentgenol. 1979;133:1051-1055. Gray SW, Rowe JS Jr, Skandalakis JE. Surgical anatomy of the gastroesophageal junction. Am Surg. 1979;45:575-587. Liebermann-Meffert D. The pharyngoesophageal segment: anatomy and innervation. Dis Esophagus. 1995;8:242-251. Liebermann-Meffert D, Siewert JR. Arterial anatomy of the esophagus: a review of the literature with brief comments on clinical aspects. Gullet. 1992;2:3-10. Liebermann-Meffert DM, Meier R, Siewert JR. Vascular anatomy of the gastric tube used for esophageal reconstruction. Ann Thorac Surg. 1992;54(6):1110-1115. Liebermann-Meffert DM, Walbrun B, Hiebert CA, et al. Recurrent and superior laryngeal nerves: a new look with implications for the esophageal surgeon. Ann Thorac Surg. 1999;67:217-223. Physiology Barlow AP, DeMeester TR, Ball CS, et al. The significance of the gastric secretory state in gastroesophageal reflux disease. Arch Surg. 1989;124:937-940. DeMeester TR, Lafontaine E, Joelsson BE, et al. The relationship of a hiatal hernia to the function of the body of the esophagus and the gastroesophageal junction. J Thorac Cardiovasc Surg. 1981;82(4):547-558. Helm JF, Dodds WJ, Pelc LR, Palmer DW, Hogan WJ, Teeter BC. Effect of esophageal emptying and saliva on clearance of acid from the esophagus. N Engl J Med. 1984;310:284-288. Joelsson BE, DeMeester TR, Skinner DB, LaFontaine E, Waters PF, O’Sullivan GC. The role of the esophageal body in the antireflux mechanism. Surgery. 1982;92:417-424. Johnson LF, DeMeester TR. Evaluation of elevation of the head of the bed, bethanechol, and antacid foam tablets on gastroesophageal reflux. Dig Dis Sci. 1981;26:673-680. Kahrilas PJ, Dodds WJ, Hogan WJ. Effect of peristaltic dysfunction on esophageal volume clearance. Gastroenterology. 1988;94:73-80. McCallum RW, Berkowitz DM, Lerner E. Gastric emptying in patients with gastroesophageal reflux. Gastroenterology. 1981;80:285-291. Mittal RK, Lange RC, McCallum RW. Identification and mechanism of delayed esophageal acid clearance in subjects with hiatus hernia. Gastroenterology. 1987;92:130-135. Rao SSC, Madipalli RS, Mujica VR, et al. Effects of age and gender on esophageal biomechanical properties and sensation. Am J Gastroenterol. 2003;98:1688-1695. Tseng D, Rizvi AZ, Fennerty MB, et al. Forty-eight-hour pH monitoring increases sensitivity in detecting abnormal esophageal acid exposure. J Gastrointest Surg. 2005;9:1043-1051; discussion 1051. Zaninotto G, DeMeester TR, Schwizer W, et al. The lower esophageal sphincter in health and disease. Am J Surg. 1988;155:104-111. Assessment of Esophageal Function Adamek RJ, Wegener M, Weinbeck M, Gielen B. Long-term esophageal manometry in healthy subjects: evaluation of normal values and influence of age. Dig Dis Sci. 1994;39:2069-2073. Barish CF, Castell DO, Richter JE. Graded esophageal balloon distention: a new provocative test for non-cardiac chest pain. Dig Dis Sci. 1986;31:1292-1298. Battle WS, Nyhus LM, Bombeck CT. Gastroesophageal reflux: diagnosis and treatment. Ann Surg. 1973;177:560-565. Bernstein IM, Baker CA. A clinical test for esophagitis. Gastroenterology. 1958;34:760-781. DeMeester TR, Johnson LF, Joseph GJ, Toscano MS, Hall AW, Skinner DB. Patterns of gastroesophageal reflux in health and disease. Ann Surg. 1976;184(4):459-470. DeMeester TR, Wang CI, Wernly JA, et al. Technique, indications and clinical use of 24-hour esophageal pH monitoring. J Thorac Cardiovasc Surg. 1980;79:656-670. Dodds WJ. Current concepts of esophageal motor function: clinical implications for radiology. AJR Am J Roentgenol. 1977;128:549-561. Fein M, Fuchs KH, Bohrer T, et al. Fiberoptic technique for 24-hour bile reflux monitoring. Standards and normal values for gastric monitoring. Dig Dis Sci. 1996;41:216-225. Fuchs KH, DeMeester TR, Albertucci M. Specificity and sensitivity of objective diagnosis of gastroesophageal reflux disease. Surgery. 1987;102:575-580. Iascone C, DeMeester TR, et al. Barrett’s esophagus: functional assessment, proposed pathogenesis, and surgical therapy. Arch Surg. 1983;118:543-549. Johnson LF, DeMeester TR. Development of 24-hour intraesophageal pH monitoring composite scoring. J Clin Gastroenterol. 1986;8(suppl 1):52-58. Johnson LF, DeMeester TR. Twenty-four-hour pH monitoring of the distal esophagus: a quantitative measure of gastroesophageal reflux. Am J Gastroenterol. 1974;62(4):325-332. Kauer WK, Burdiles P, Ireland A, et al. Does duodenal juice reflux into the esophagus in patients with complicated GERD? Evaluation of a fiberoptic sensor for bilirubin. Am J Surg. 1995;169:98-103. 1091 CHAPTER 25 ESOPHAGUS AND DIAPHRAGMATIC HERNIA Although conceptually appealing, preservation of vagal nerve integrity or the gastric reservoir function after vagal sparing esophagectomy only recently has been validated. Banki and associates compared patients undergoing vagal sparing esophagectomy to those with conventional esophagectomy and colon or gastric interposition. This study showed that vagal sparing esophagectomy preserved gastric secretion, gastric emptying, meal capacity, and body mass index, compared to esophagogastrectomy with colon interposition or standard esophagectomy with gastric pull-up. Vagal sparing esophagectomy patients functioned, for the most part, similarly to normal subjects, allowing them to eat a normal meal, free of dumping or diarrhea. These results indicate that the vagal-sparing esophagectomy procedure does indeed preserve the vagal nerves, and it may be considered in the treatment of benign and early malignant lesions requiring esophagectomy.