Saturated & Unsaturated Solutions, Solubility Worksheet
advertisement

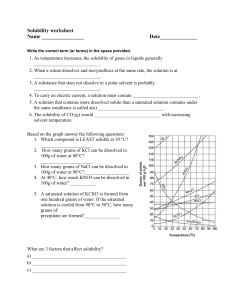
Solutions can be classified as saturated or unsaturated. A saturated solution It’s a solution in which no more solute can dissolve at that temperature (It contains the maximum quantity of solute that dissolves at that temperature) An unsaturated solution It’s a solution in which more solute can dissolve at that temperature (it contains less than the maximum amount of solute that can dissolve at that particular temperature) • What is the solubility of a substance? It is the maximum mass of a substance that dissolves in 100g of water at a particular temperature e.g. maximum mass of sodium chloride that dissolve in 100g of water at 30oC is 38g. So the solubility of sodium chloride in water at 30oC is 38g per 100g of water Solubility curve • It’s a curve that shows the solubility of a substance at different temperatures of the solvent. X axis – temperature Y axis – solubility in g per 100g of water • The points on the curve shows the maximum amount of the substance that dissolve in 100 g of water at that temperature Solubility of solids with temperature • More solid can dissolve in a solvent at higher temperatures • If a saturated solution is heated it becomes unsaturated because more solute could dissolve when temperature is increased. • If a saturated solution is cooled down, some solute crystallises out of the solution because less solute could dissolve when temperature is decreased. Solubility of gases with temperature • less gas can dissolve in a solvent, at higher temperatures. • As the temperature rises, gases become less soluble in water e.g: When warm water is released into water bodies, it increases the temperature of water and decreases the amount of dissolved oxygen. Fish die due to lack of oxygen Temperature (oC) 0 20 40 60 80 90 3 8 14 24 38 44 Solubility/ g per 100g of water Potassium chloride dissolves in water. Use the data given below to plot a solubility curve for potassium chloride Using your graph, answer the following questions. i) What is the relationship between the temperature and the solubility of potassium chloride? ii) What mass of potassium chloride dissolves in 100g of water at 50oC? 50 45 40 35 30 25 20 15 10 5 0 0 10 20 30 40 50 60 70 80 90 100 iii) What is the lowest the lowest temperature at which 30g of potassium chloride will dissolve in 100g of water? iv) If a saturated solution of potassium chloride in 100g of water at 60oC, is cooled down to 10oC, how much potassium chloride will crystallise? v) If a saturated solution of potassium chloride in 100g of water at 50oC is heated up to 80oC, how much more potassium chloride can dissolve?