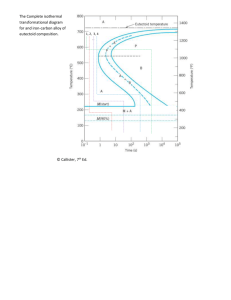
Why fine grained structure is harder than coarse grain structure? A-7: The smaller the grain size, the more frequent is the pile up of dislocations. With decrease in grain size, the mean distance of a dislocation can travel decreases, and soon starts pile up of dislocations at grain boundaries. This leads to increase in yield strength of the material. What are hypoeutectoid and hypereutectoid steels, explain. A-17: Hypoeutectoid Steel Plain carbon steels in which carbon percentage is less than 0.8% are called hypoeutectoid steel. Hypereutectoid Steel Plain carbon steels in which carbon percentage is more than 0.8% are called hypoeutectoid steel. What is the difference between martempering and austempering? A-21: Martempering is a modified quenching procedure used to minimize distortion and cracking that may develop during uneven cooling of the heat-treated material. It involves cooling the austenized steel to temperature just above Ms temperature, holding it there until temperature is uniform, followed by cooling at a moderate rate to room temperature before austenite-to-bainite transformation begins. The final structure of martempered steel is tempered Martensite. Austempering is different from martempering in the sense that it involves austenite-to bainite transformation. Thus, the structure of austempered steel is bainite. Advantages of austempering are improved ductility; decreased distortion and disadvantages are need for special molten bath; process can be applied to limited number of steels. What are the different phases in an iron-carbon diagram? The different phases in an iron-carbon diagram include austenite, pearlite, bainite, martensite, ferrite, and cementite. What is austenite and where is it found in the Fe-C diagram? Austenite is a face-centered cubic crystal structure that is found above the critical temperature (Ac3) in the Fe-C diagram. It is the highest temperature phase that contains both iron and carbon in solid solution. What is pearlite and where is it found in the Fe-C diagram? Pearlite is a two-phase microstructure composed of alternating layers of ferrite and cementite. It is found in the range of temperatures between the critical temperature (Ac3) and the lower critical temperature (Ac1). What is bainite and where is it found in the Fe-C diagram? Bainite is a mixture of ferrite and cementite that forms at intermediate temperatures between the critical temperature (Ac3) and the martensite start temperature (Ms). What is martensite and where is it found in the Fe-C diagram? Martensite is a highly stressed, body-centered tetragonal crystal structure that forms at very low temperatures. It is found in the range of temperatures below the martensite start temperature (Ms). What is ferrite and where is it found in the Fe-C diagram? Ferrite is a body-centered cubic crystal structure that is found at low temperatures in the Fe-C diagram. It is a pure iron phase with no carbon in solid solution. What is cementite and where is it found in the Fe-C diagram? Cementite is a hard, brittle iron-carbon compound that is found in the Fe-C diagram as a separate phase from ferrite. It forms as a result of the precipitation of excess carbon from the austenitic solid solution. How does carbon content affect the microstructure in an iron-carbon alloy? The carbon content in an iron-carbon alloy determines the types of phases and microstructures that will form. Increasing carbon content can lead to the formation of phases such as pearlite, bainite, and martensite, while decreasing carbon content can result in the formation of ferrite. What is the significance of the critical temperatures (Ac3, Ac1, and Ms) in the Fe-C diagram? The critical temperatures (Ac3, Ac1, and Ms) in the Fe-C diagram are important for determining the microstructures that will form during heating and cooling processes. The critical temperature (Ac3) marks the highest temperature at which austenite can exist, while the lower critical temperature (Ac1) marks the beginning of pearlite formation. The martensite start temperature (Ms) marks the beginning of martensite formation. How is the Fe-C diagram used in metallurgy and materials science? The Fe-C diagram is widely used in metallurgy and materials science as a tool for understanding the relationships between heating and cooling processes, carbon content, and microstructures in iron-carbon alloys. It is used to predict the types of microstructures that will form during heat treatment, which is critical in the production of high-strength steels. Can the Fe-C diagram be used for alloys other than iron-carbon alloys? The Fe-C diagram is specifically designed for iron-carbon alloys and cannot be used for alloys containing other elements. For alloys containing other elements, similar diagrams such as the nickel-chromium (Ni-Cr) or aluminum-copper (Al-Cu) diagrams can be used to predict microstructures. Can the Fe-C diagram predict the mechanical properties of iron-carbon alloys? The Fe-C diagram can provide information about the microstructure of iron-carbon alloys, which can be used to make predictions about the mechanical properties of the material. For example, the presence of martensite in an iron-carbon alloy can indicate that the material has high strength and hardness, while the presence of ferrite can indicate that the material has lower strength and hardness. However, it is important to note that the Fe-C diagram provides only a general understanding of the microstructure and mechanical properties, and that more detailed analysis and testing may be necessary to fully understand the properties of a specific material. How is the Fe-C diagram affected by cooling rate? The Fe-C diagram assumes a specific cooling rate, usually an isothermal cooling rate. If the cooling rate is different, the actual microstructure that forms can be different from what is predicted by the Fe-C diagram. For example, if the cooling rate is increased, the amount of martensite that forms may increase, leading to higher strength and hardness. Conversely, if the cooling rate is decreased, the amount of ferrite and pearlite that form may increase, leading to lower strength and hardness. How is the Fe-C diagram affected by alloying elements? The presence of alloying elements can have a significant impact on the Fe-C diagram. The addition of certain elements, such as nickel, chromium, and molybdenum, can shift the critical temperatures and change the amount and type of phases that form. This can have a significant impact on the mechanical properties of the material, such as strength and toughness, and must be taken into account when using the Fe-C diagram to predict microstructures and properties. What is the difference between the eutectoid reaction and the peritectic reaction in the Fe-C diagram? The eutectoid reaction in the Fe-C diagram occurs at the eutectoid composition, which is 0.8% carbon. At this composition, austenite transforms into a mixture of pearlite and cementite, which are both comprised of ferrite and iron carbide (Fe3C). The peritectic reaction, on the other hand, occurs at a specific temperature and composition, and involves the formation of a new solid phase in addition to the liquid phase. In the Fe-C diagram, the peritectic reaction occurs at the peritectic point, which is located near the upper critical temperature. What is the role of heat treatment in the Fe-C diagram? Heat treatment plays a critical role in the Fe-C diagram, as it can be used to manipulate the microstructure of iron-carbon alloys. By controlling the heating and cooling rates, it is possible to produce specific microstructures, such as martensite, pearlite, or bainite, which can be used to optimize the mechanical properties of the material. The Fe-C diagram can be used to determine the temperatures and cooling rates needed to produce a desired microstructure. How is fatigue different from creep? Creep is the slow and continuous deformation of a material under a constant stress, usually at high temperatures. Fatigue is the progressive and localized damage of a material under cyclic or fluctuating stress, usually at low or moderate temperatures. What is the difference between annealing, quenching, and tempering? Annealing: Annealing is a heat treatment process used to soften metals and relieve internal stresses. It involves heating the metal to a specific temperature and then slowly cooling it. This process improves ductility and reduces hardness, making the metal more workable. Quenching: Quenching is the rapid cooling of a heated metal by immersing it in a cooling medium, such as oil or water. This process increases hardness and strength but may also introduce brittleness. Tempering: Tempering follows quenching and involves reheating the metal to a lower temperature. This relieves some of the excessive hardness from quenching, enhancing toughness and reducing brittleness. The exact temperature and time during tempering can be adjusted to achieve the desired balance of strength and toughness. What is the purpose of metallography, and how is it conducted? Metallography is a crucial technique in metallurgy used to study and analyze the microstructure of metallic materials. Its primary purpose is to understand the arrangement and distribution of phases, grains, inclusions, and defects within a metal sample. This information is vital for assessing the material's properties and performance. Metallography involves the following key steps: Sample Preparation: A representative sample is cut from the metal and then mounted in a resin or epoxy to maintain its structure. It is crucial to ensure that the sample's surface is flat and free from any damage or deformities. Grinding and Polishing: The mounted sample is ground using progressively finer abrasives to create a smooth and flat surface. After grinding, the sample is polished to obtain a mirror-like finish. Etching: Etching is performed using specific chemical reagents that selectively attack certain phases or grains within the metal. This enhances the visibility of microstructural features under a microscope. Microscopic Examination: The prepared sample is examined under an optical or electron microscope, depending on the level of detail required. The microstructure is then analyzed and documented. Image Capture: High-quality images of the microstructure are captured, which can be used for further analysis and documentation. Metallography provides valuable information about grain size, grain boundaries, phase composition, and the presence of defects like cracks or inclusions. This data helps metallurgists assess material quality, predict mechanical properties, and diagnose any issues related to manufacturing or service conditions. Q: What is heat treatment? A: Heat treatment is a process used to alter the physical, mechanical, and/or chemical properties of a material. This is achieved by heating and cooling the material in a controlled manner to achieve the desired result. Q: What are the main objectives of heat treatment? A: The main objectives of heat treatment are to improve the material’s hardness, toughness, ductility, and/or corrosion resistance, and to reduce internal stresses and residual stresses. Q: What are the different types of heat treatment processes? A: Some of the most common heat treatment processes include annealing, normalizing, hardening, tempering, quenching, and case hardening. Q: What is annealing? A: Annealing is a heat treatment process that is used to soften a material and reduce its hardness. This is accomplished by heating the material to a specific temperature for a specific amount of time and then slowly cooling it. Q: What is normalizing? A: Normalizing is a heat treatment process that is used to improve the material’s toughness and homogeneity. It is similar to annealing, but the cooling process is faster. Q: What is hardening? A: Hardening is a heat treatment process that is used to increase the hardness of a material. It is accomplished by heating the material to a specific temperature, holding it at that temperature for a specific amount of time, and then rapidly cooling it. Q: What is tempering? A: Tempering is a heat treatment process that is used to reduce the hardness of a material and improve its toughness. This is accomplished by heating the material to a specific temperature for a specific amount of time and then slowly cooling it. Q: What is quenching? A: Quenching is a rapid cooling process that is used to harden a material. It is performed after the material has been heated to a specific temperature, and it helps to reduce the grain size of the material and improve its hardness. Q: What is case hardening? A: Case hardening is a heat treatment process that is used to improve the surface hardness of a material while maintaining its toughness in the core. This is accomplished by first carburizing the surface of the material and then quenching it. Q: What is the importance of heat treatment in the manufacturing process? A: Heat treatment is an important step in the manufacturing process because it can significantly improve the material’s properties and performance. By changing the microstructure of the material through heat treatment, the material can be made harder, stronger, more wear-resistant, and more corrosion-resistant, which can improve its overall performance in the intended application. Q: What are the factors that influence the heat treatment process? A: The factors that influence the heat treatment process include the type of material being treated, the desired properties, the heating and cooling rate, the time at temperature, the cooling method, and the environment in which the treatment is performed. Q: What are the factors that determine the selection of a heat treatment process? A: The selection of a heat treatment process depends on the desired properties and the type of material being treated. The process may also be influenced by the size and shape of the material, the surface condition, and the cost. Q: What are the most commonly used heating and cooling methods in heat treatment? A: The most commonly used heating methods in heat treatment include induction heating, resistance heating, and gas heating. The most commonly used cooling methods include air cooling, oil cooling, water cooling, and cryogenic cooling. Q: What is hardening and what is it used for? A: Hardening is a heat treatment process where a material is heated to a temperature above its critical range and then rapidly cooled, typically by quenching in oil, water, or air. The rapid cooling rate promotes the formation of a hard, brittle microstructure known as martensite, which gives the material improved hardness and strength. Hardening is used to improve the wear resistance and service life of tools, machinery components, and other high-stress parts. Q: What is normalizing and what is it used for? A: Normalizing is a heat treatment process where a material is heated to an elevated temperature and then cooled in air. The purpose of normalizing is to improve the material’s mechanical properties and uniformity of its microstructure. Normalizing is often used to refine the grain structure of steel and to homogenize the material prior to hardening. Q: What is tempering and what is it used for? A: Tempering is a heat treatment process where a hardened material is reheated to a temperature below its critical range and then cooled, typically in air. The purpose of tempering is to reduce the brittleness of the material that may have been introduced during hardening and to improve its toughness and ductility. Tempering is used to improve the serviceability of hardened parts and to reduce the risk of failure due to brittle fracture. Q: What is the difference between annealing and normalizing? A: Annealing is a heat treatment process where a material is heated to a temperature above its critical range and then cooled slowly in order to achieve a soft and homogeneous microstructure. Normalizing, on the other hand, is a heat treatment process where a material is heated to an elevated temperature and then cooled in air. The purpose of normalizing is to improve the material’s mechanical properties and uniformity of its microstructure. Q: What is the difference between annealing and tempering? A: Annealing is a heat treatment process that involves heating a material to a temperature above its critical range and then cooling it slowly in order to achieve a soft and homogeneous microstructure. Tempering, on the other hand, is a heat treatment process where a hardened material is reheated to a temperature below its critical range and then cooled, typically in air. The purpose of tempering is to reduce the brittleness of the material that may have been introduced during hardening and to improve its toughness and ductility. Q: What is the difference between quenching and tempering? A: Quenching is a heat treatment process where a material is rapidly cooled from an elevated temperature in order to produce a hard, brittle microstructure. Tempering, on the other hand, is a heat treatment process where a hardened material is reheated to a temperature below its critical range and then cooled, typically in air. The purpose of tempering is to reduce the brittleness of the material that may have been introduced during hardening and to improve its toughness and ductility. Q. Show Temp. Range for different heat treatment processes on Iron carbon diagram. Q. Draw Iron Carbon Diagram and mark all reactions on it. or Draw iron carbon diagram and show all microconstiuents/phases present on it. Ans. Characteristics of phases appeared in Fe-Fe3C phase diagram The Fe-Fe3C phase diagram, also known as the iron-carbon phase diagram, describes the phases that can appear in iron-carbon alloys as a function of temperature and carbon content. The main phases that appear in this diagram are: Ferrite (α): Ferrite is a body-centered cubic (BCC) phase of iron that is stable at low temperatures. It can dissolve a small amount of carbon, up to 0.022% at 727°C. Austenite (γ): Austenite is a face-centered cubic (FCC) solid solution of carbon in iron. It is stable at high temperatures and can dissolve up to 2.11% carbon at 1147°C. Cementite (Fe3C): Cementite is a compound that consists of iron and carbon in a fixed ratio of 6.67% carbon. It has an orthorhombic crystal structure and is a hard and brittle phase. Pearlite: Pearlite is a two-phase microstructure consisting of alternating layers of ferrite and cementite. It forms when austenite is cooled rapidly to a temperature between 727°C and 550°C, which is in the eutectoid region of the phase diagram. Martensite: Martensite is a metastable phase that forms when austenite is rapidly quenched to a temperature below the eutectoid temperature. It has a highly distorted BCC structure and is extremely hard and brittle. Liquid: At temperatures above the melting point of iron (1538°C), the alloy exists as a liquid phase. These phases and their compositions can be used to predict the mechanical and physical properties of iron-carbon alloys. Ferrite (α) It is an interstitial solid solution of a small amount of carbon dissolved in α iron. The maximum solubility is 0.025%C at 723ºC and it dissolves only 0.008%C at room temperature. It is the softest structure that appears on the diagram. Ferrite is ferromagnetic at low temperatures but loses its magnetic properties with the rise of temperatures with major loss at curies temperatures, 768ºC and above this temperature, it becomes non magnetic (paramagnetic). The crystal structure of ferrite (α) is B.C.C Tensile strength – 245 Mpa, Yield strength 118 Mpa Elongation – 40-50% in 2 in. Hardness – 95 VPN Cementite (Fe3C) Cementite or iron carbide, chemical formula Fe3C, contains 6.67%C by weight and it is a metastable phase. It is typically hard and brittle interstitial compound of low tensile strength (35 Mpa) but high compressive strength and high hardness ~800VPN. It is the hardest structure that appears on the diagram. It has a complex orthorhombic crystal structure with 12 iron atoms and 4 carbon atoms per unitcell. It is slightly ferromagnetic up to 210ºC and paramagnetic above it. Melting point around 1227ºC. Pearlite (α+Fe3C) Pearlite is the eutectoid mixture containing 0.80 %C and is formed at 723ºC on very slow cooling. It is very fine platelike or lamellar mixture of ferrite and cementite. The fine fingerprint mixture called pearlite is shown in below figure. The weight % of these two phases are thus in ratio 8:1 Tensile strength – 120,000 psi or 825 Mpa Elongation – 20 percent in 2 in. Hardness – HRC 20, HRB 95-100, or BHN 250-300 Austenite (γ) It is an interstitial solid solution of a small amount of carbon dissolved in γ iron and has FCC crystal structure. The maximum solubility is 2.1%C at 1147ºC. Austenite is soft, ductile tough and malleable (FCC structure) and non magnetic (paramagnetic). Steels are commonly rolled and forged above about 1100ºC when they are in austenite state due to its high ductility and malleability, which is also due to its FCC structure. Tensile strength – 150,000 psi or 1035 Mpa Elongation – 10% in 2 in. Hardness – 395 VPN and Toughness is high. Ledeburite (γ+Fe3C) Ledeburite is the eutectic mixture of austenite and cementite. It contains 4.3%C and is formed at 1147ºC Structure of ledeburite contains small islands of austenite are dispersed in the carbide phase. Not stable at room temperature The pearlite is resolved in some regions where the sectioning plane makes a glancing angle to the lamellae. The ledeburite eutectic is highlighted by the arrows. At high temperatures this is a mixture of austenite and cementite formed from liquid. The austenite subsequently decomposes to pearlite. Ferrite (δ) Interstitial solid solution of carbon in iron of body centered cubic crystal structure. (δ iron ) of higher lattice parameter (2.89Å) having solubility limit of 0.09 wt% at 1495°C with respect to austenite. The stability of the phase ranges between 13941539°C. It is a high temperature phase and is a high temperature manifestation of α ferrite. This is not stable at room temperature in plain carbon steel. However it can be present at room temperature in alloy steel specially duplex stainless steel. Q. Draw TTT diagram and mark all microconstitutents on it. Also need to cover Edge dislocation vs screw dislocation Schottky vs frenkel defect Burger vector