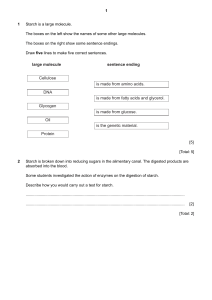
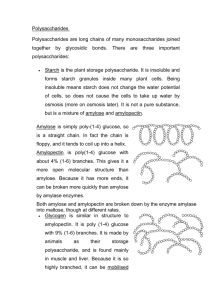
ASSIGNMENT 1 1a. Explain why glucose is a reducing sugar and fructose is not. Glucose is classified as a reducing sugar because it has a free aldehyde or ketone group that can donate electrons to other compounds. In the case of glucose, the aldehyde group is located on the first carbon atom of the sugar molecule. This aldehyde group can undergo a chemical reaction known as a redox reaction, where it donates electrons to another compound, thereby reducing it. This property of glucose makes it an important component in various biological processes, such as glycolysis, where it serves as a source of energy for cells. Fructose, on the other hand, is not classified as a reducing sugar because it lacks a free aldehyde or ketone group. Instead, fructose has a ketone group on the second carbon atom of the sugar molecule. This ketone group is not reactive in the same way as an aldehyde group, and therefore fructose cannot donate electrons to other compounds in the same manner as glucose. As a result, fructose is not able to undergo the same redox reactions as glucose and is therefore not considered a reducing sugar. b. Describe the composition of starch highlighting the differences in Amylose and Amylopectin (7) Amylose is a linear polymer of glucose units linked together by alpha-1,4 glycosidic bonds. It is a long, straight chain molecule that is soluble in hot water and forms a gel-like substance when it is cooled. Amylose contributes to the thickness and creaminess of various food products, such as puddings and sauces. It is also responsible for the formation of the blue-black colour when iodine is added to a starch solution. Amylose typically makes up about 20-30% of starch Amylopectin is a branched polymer of glucose units linked together by alpha-1,4 and alpha1,6 glycosidic bonds. It has a highly branched structure, with multiple side chains branching off from the main chain. Amylopectin is insoluble in water and does not form gels like amylose. Instead, it contributes to the sticky and cohesive properties of starch, making it ideal for use as a thickening agent in various food products The difference in structure between amylose and amylopectin is key to their different properties and functionalities. While amylose is a long, linear chain that forms gels and contributes to the creaminess of food products, amylopectin is a highly branched structure that provides viscosity and texture to food products. Both components play important roles in the cooking and processing of starch-based foods, as well as in the storage and release of energy in plants. c. Describe the difference in structure and characteristics between cellulose and starch. One of the main differences between cellulose and starch lies in their structural composition. Cellulose is a polysaccharide made up of β-glucose monomers linked together by β-1,4glycosidic bonds. This results in a linear and rigid structure, with hydrogen bonding between adjacent glucose molecules contributing to the formation of microfibrils. These microfibrils are further arranged into larger microfibrils, creating a strong and resistant network that forms the main component of plant cell Starch is also a polysaccharide composed of glucose units, but in this case, the glucose molecules are linked by α-1,4-glycosidic bonds. Additionally, starch can contain branching points where α-1,6-glycosidic bonds connect the glucose chains. This branching gives starch a more flexible and helical structure, making it easily digestible by enzymes such as amylase Another important difference between cellulose and starch is in their function and characteristics. Cellulose is a structural component of plant cell walls, providing rigidity and support to the plant cell. Due to its linear and rigid structure, cellulose is insoluble in water and resistant to degradation by most enzymes, making it indigestible for many organisms, including humans. However, cellulose serves as an important dietary fiber that aids in digestion and promotes gut health Starch serves as a storage form of energy in plants, storing glucose for later use. Starch is found in various plant tissues such as seeds, tubers, and grains, where it can be readily broken down into glucose by enzymes to produce energy. This makes starch a valuable source of nutrition for humans and animals. 2. Illustrate the formation of a triglyceride molecule from glycerol and fatty acids References: 1. Jane, J. L., & Robyt, J. F. (1984). Structure of amylose and amylopectin films. Carbohydrate Research, 128, 111-123. 2. Tester, R. F., & Karkalas, J. (2002). Starch composition, fine structure and architecture. Journal of Cereal Science, 39(2), 151-165. 3. Lodish H, Berk A, Zipursky SL, et al. Molecular Cell Biology. 4th edition. New York: W. H. Freeman; 2000. Section 14.5, Polysaccharides: The Peptidoglycan of Bacterial Cell Walls and the Murein Sacculus of Yeast.