ABSTRACT
Background: Postoperative acute kidney injury (AKI) is common following cardiac surgery.
Body mass index may be an amenable variable by representing the summation of the nutritional
and fluid status. However, the predictive role of perioperative BW changes in CS patients with
severe postoperative AKI is never explored. This study aimed to evaluate this association.
Methods & Materials: We performed prospective cross-sectional research on the effect of
body mass index on the kidney function of patients undergoing coronary artery bypass grafting
and valve replacement surgery. This research was performed under the permission of the board
of the Punjab Institute of Cardiology. Our research examined 107 patients in the operative ward
of PIC undergoing open-heart cardiopulmonary bypass surgery.
Results: Our study has identified many perioperative variables that work synergistically with the
body mass index of the patient, and are significantly important in stratifying risk for the
development of post-operative acute kidney failure.
Key Words:
Body Mass Index; Acute Kidney Injury; coronary artery bypass grafting: Overweight; Normal
weight; isolated valve surgeries; NYHA class; Diuretics; Urea; Creatinine; Urine Output;
Bypass time; Cross clamp time; Dialysis;
1
1.0: INTRODUCTION
This study aimed to determine whether weight is an independent predictor of postoperative
renal insufficiency in patients undergoing cardiac surgeries, including patients undergoing
isolated coronary artery bypass grafting (CABG), isolated valve surgeries, and combined
CABG and valve surgeries. We also investigated the possible association between obesity and
increased severity of postoperative renal failure (that is, postoperative renal failure requiring
in-hospital dialysis).
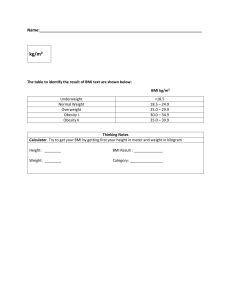
1.1 Body Mass Index (BMI) and its categories:
According to Weir C. et al. (2019), body mass index or BMI is a statistical index using a
person's weight and height to provide an estimate of body fat in males and females of any age.
It is calculated by taking a person's weight, in kilograms, divided by their height, in meters
squared, or BMI = weight (in kg)/ height^2 (in m^2). The number generated from this equation
is then the individual's BMI number.
The National Institute of Health (NIH) now uses BMI to define a person as underweight,
normal weight, overweight, or obese instead of traditional height vs. weight charts. These
classifications for BMI are in use by the NIH and the World Health Organization (WHO) for
White, Hispanic, and Black individuals.
2
Table no# 1: Categories of BMI
Categories
Range of BMI
Severely underweight
BMI less than 16.5kg/m^2
Underweight
BMI less than 18.5 kg/m^2
Normal weight
BMI greater than or equal to 18.5 to 24.9
kg/m^2
Overweight
BMI greater than or equal to 25 to 29.9
kg/m^2
Obesity
BMI greater than or equal to 30 kg/m^2
1.2 Underweight patients:
According to Robbert H. et al. (2005) even after rigorous matching of body size cohorts to
normal size counterparts, we found that large deviations from normal (very small and very
obese) are independently associated with increased postoperative morbidity and worse longterm survival. Small patients also exhibited greater operative death, which is perhaps linked to
increased on-pump hemodilution, transfusions, and associated complications. We suggest that
these effects in small patients are potentially modifiable by changes in current CPB practice.
Current CABG demographic trends for our practice show that very obese is the only growing
CABG subpopulation and this occurs at the expense of the normal cohort. The very obese
cohort was also associated—even after accounting for other comorbidities—with the most
perioperative morbidity and the highest relative death hazard after the first year.
1.3 Obesity an epidemic:
According to David et al (2007) obesity is now reaching epidemic proportions in both
developed and developing countries and is affecting not only adults but also children and
adolescents. Over the last 20 years, obesity has become the most prevalent nutritional problem
in the world, eclipsing undernutrition and infectious disease as the most significant contributor
3
to ill health and mortality. It is a key risk factor for many chronic and noncommunicable
diseases.
Since the prevalence of comorbid factors is itself increased by morbid obesity, then the risks
we report for very obese status may underestimate its true effects. These data in CABG
outcomes are consistent with the effects of obesity in the general adult population and
underscore the importance of reversing the well-documented obesity epidemic from a coronary
artery disease perspective.
There is compelling evidence that Obesity is associated with a cluster of risk factors for health
problems, including type 2 diabetes, hypertension, dyslipidemia, coronary artery disease,
stroke, osteoarthritis, and certain forms of cancers.
1.4 Kidney diseases and obesity
A study by Amann et al. (2013) showed that apart from cardiovascular disease kidney diseases
also have been shown to be associated with obesity. Epidemiologic studies have indicated that
obese patients, in general, are at an increased risk for developing acute kidney injury (AKI)
due to their disproportionately higher burden of comorbidities and due to underlying structural
changes that occur in the kidneys of obese patients irrespective of the presence or absence of
diabetes, arterial hypertension, and other comorbidities.
According to Chagnac et al. (2000), excess body weight is associated with functional and
structural renal changes, such as increased glomerular filtration rate (GFR), renal plasma flow
(RPF), and urinary albumin excretion.
According to Katie E. et al. (2015) acute kidney injury (AKI) occurs in approximately 30% of
patients undergoing cardiac surgery and significantly increases short- and long-term morbidity
and mortality.
4
According to Karkouti K. et al (2015) preoperative anemia, intraoperative anemia, and RBC
transfusion on the day of surgery are interrelated risk factors for AKI after cardiac surgery.
Targeting these risk factors may reduce the burden of AKI.
By Kandler et al. (2014) research, more than 28% of the patients undergoing elective or
subacute cardiac surgery developed AKI in this contemporary cohort. Furthermore, acute
kidney injury was an independent predictor of increased mortality irrespective of the
perioperative risk factors.
1.5 Acute Kidney Diseases (AKD) and classification:
According to Ricci et al. (2011), the term ‘acute kidney injury (AKI) is currently recognized
as the preferred nomenclature for the complex clinical syndrome formerly known as acute renal
failure (ARF). This transition in terminology also serves to emphasize that the spectrum of
disease is much broader than the subset of patients who experience renal failure requiring
dialysis treatment. AKI occurs in a variety of settings and has clinical manifestations ranging
from a minimal elevation in serum creatinine levels to anuric renal failure.
AKI exists along a continuum of diseases: the acute decline in kidney function is often
secondary to an injury that causes functional or structural changes in the kidneys. As the
severity of the underlying renal injury increases, the risk of an unfavorable outcome rises.
1.6 The RIFLE criteria:
The definition of RIFLE criteria, and uses the following categories: ‘at Risk’ is the least severe
category of AKI, followed by ‘Injury’, ‘Failure’, ‘Loss’, and ‘End-stage renal disease’. This
definition was intended to establish the presence or absence of clinical AKI in a given patient
or situation and to describe the severity of this syndrome.
Table 2 | RIFLE Classification and staging systems for AKI
5
System
Serum creatinine criteria
Urine output criteria
Risk
Serum creatinine increase to
<0.5ml/kg/h for 6h
1.5-fold OR GFR decrease
>25% from baseline
Injury
Serum creatinine increase to
<0.5ml/kg/h for 12h
2.0-fold OR GFR decrease
>50% from baseline
Failure
Serum creatinine increase to
Anuria for 12h
3.0-fold OR GFR decrease
>75% from baseline OR
serum creatinine ≥354μmol/l
(≥4mg/dl) with an acute
increase of at least 44μmol/l
(0.5mg/dl)
1.7 AKIN criteria:
The AKIN criteria state that “diagnosis based on the urine criterion alone will require exclusion
of urinary tract obstructions that reduce urine output or of other easily reversible causes of
decreased urine output” and that application of the diagnostic criteria “should be used in the
context of the clinical presentation and following adequate resuscitation when applicable”.
6
Table 3 | AKIN Classification and staging systems for AKI
System
Serum creatinine criteria
Urine output criteria
1
Serum creatinine increase
<0.5ml/kg/h for 6h
≥26.5μmol/l (≥0.3mg/dl) OR
increase to 1.5–2.0-fold
from baseline
2
Serum creatinine increase
<0.5ml/kg/h for 12h
>2.0–3.0-fold from baseline
3
Serum creatinine increase
<0.3ml/kg/h for 24h OR
>3.0-fold from baseline OR
anuria for 12h OR need for
serum creatinine ≥354μmol/l RRT
(≥4.0mg/dl) with an acute
increase of at least 44μmol/l
(0.5mg/dl) OR need for RRT
1.8: Acute Kidney diseases after cardiac surgery
ACUTE KIDNEY INJURY (AKI) following cardiac surgery is a well-known complication
occurring in 1% to 56% of patients depending largely on the AKI definition used and the study
cohort. The mortality has been shown to be raised substantially, even in lower stages of AKI
such as the Acute Kidney Injury Network (AKIN) stage, which is defined as a postoperative
increase in serum creatinine (sCr) of 450% compared to baseline. The etiology of AKI is
multifactorial, and several patient-related risk factors have been found, including female
gender, diabetes, chronic obstructive pulmonary disease, peripheral vascular disease, and low
ejection fraction.
According to Mitchell et al. (2005) some of the patient-related risk factors contributing to
AKI after cardiac surgery are given below:
Female gender
Chronic obstructive pulmonary disease
7
Diabetes
Peripheral vascular disease
Renal insufficiency
Congestive heart failure
LV ejection fraction <35%
Need for emergent surgery
Cardiogenic shock (IABP)
Left main coronary disease
Some of the procedure-related risk factors contributing to AKI after cardiac surgery are given
below:
Weight
Length of CPB
Cross-clamp time
Off-pump versus on-pump
Nonpulsatile flow
Hemolysis
Hemodilution
In addition to these contributing factors to cardiovascular risk, obesity is an independent
predictor of incident AKD after cardiac surgery. There have been relatively few studies
examining obesity (based on BMI) as an independent predictor of postoperative AKI in patients
undergoing cardiac surgery with cardiopulmonary bypass (AKICPB). In this retrospective
analysis, the authors explored the association between obesity (based on BMI) and the risk of
developing AKICPB.
We conducted this study to address three questions:
1. Does BMI predict AKI after cardiac surgery?
8
2. What are the early clinical renal outcomes in normal-weight patients after undergoing
cardiac surgery?
3. What are the early clinical renal outcomes in overweight patients after undergoing
cardiac surgery?
9
2.0: LITERATURE REVIEW
Kristian K. et al. (2014) present a study conducted on an up-to-date cohort, who underwent
cardiac surgery in 2012, in which the majority of patients received ibuprofen and gentamicin.
The principal finding of the study was a high incidence of AKI after isolated CABG or CABG
in combination with valve surgery. The majority of patients had the lowest stage AKI according
to AKIN criteria. However, even AKIN stage 1 was associated with a marked increase in
mortality compared to patients without AKIN. AKIN stage 1 was present in 23% of the
patients.
The authors found a high incidence of AKI in a contemporary cohort of cardiac surgical
patients, the majority of whom received ibuprofen and gentamicin. AKI was associated
independently with higher mortality even in AKIN stage 1 regardless of the perioperative risk
evaluation. The use of nephrotoxic and potentially nephrotoxic medications, such as
gentamicin and ibuprofen, and their relation to AKI, should be investigated further.
According to the research of Barnaby C. et al. (2003), obese patients do not experience greater
morbidity and mortality than normal-weight patients after CABG after taking into account
imbalances in key prognostic factors. The overweight group did not have significantly worse
outcomes than the normal weight group for any of the adverse outcomes studied. Obese and
severely obese groups fared worse than the normal weight group only with respect to the
likelihood of staying in hospital 7 days after the operation. In contrast, underweight patients
appeared to have a higher risk of death and common complications and to recover more slowly.
Their research also concluded that obese patients with severe ischemic heart disease (e.g., the
extent of coronary disease, ejection fraction, left main stem stenosis; are relatively underrepresented among patients undergoing CABG. Conversely, there appears to be a relative
10
excess of low-weight patients with severe ischemic heart disease and important comorbidities
who needed urgent or emergency CABG.
G. Mariscalco et al. (2017) performed
a
retrospective,
observational
cohort
study
encompassing all adult cardiac surgical procedures per-formed in the United Kingdom
and Ireland. They found that overweight and obese patients had lower in-hospital mortality
compared with normal-BMI patients, whereas underweight patients had increased mortality.
This relationship was unchanged when patients with low BMI, severe chronic disease, or
severe limitation of exercise tolerance were excluded. Reductions in mortality associated with
increasing BMI class were greater in older patients and those with clinical complications of
obesity.
The relationship between obesity and secondary outcomes demonstrated heterogeneity;
obesity was associated with a reduction of primarily ischemic complications such as
low cardiac output and stroke but not RRT or infections. In a systematic review of 27
studies that included patients from 13 countries, we observed similar results. Subgroup and
metaregression analyses also demonstrated greater reductions in mortality associated with
obesity in the elderly and in patients with coronary artery disease.
M. Kuduvalli et al. (2002) research has shown that obesity is not a risk factor for in-hospital
mortality, re-exploration for bleeding, post-operative cerebrovascular accidents and renal
failure. This is in concurrence with previously published studies. They could not find an
association between obesity and perioperative myocardial infarction. They identified a
significant increase in the duration of post-operative mechanical ventilation for severely obese
patients, who were 2.74 times more likely to be ventilated for more than 48 h. This could be
due to impaired respiratory function as a result of relatively decreased vital capacity and a
prolonged depression of respiratory drive due to slow release of anesthetic agents stored in
fatty tissues into the bloodstream.
11
There was a significant increase in the incidence of post-operative atrial arrhythmia in both the
obese categories in our study, the association being more prominent in the severely obese
group.
Obesity increases oxidative stress, endothelial dysfunction, and inflammation, but the effect of
obesity on postoperative AKI is not known.
Frederic T. et al. (2012) reported that BMI is an independent risk factor for AKI after cardiac
surgery. In addition, this study is the first to report that an intraoperative marker of oxidative
stress (plasma concentrations of F2-isoprostanes) predicts AKI after cardiac surgery.
Their r data suggest that the association between obesity and AKI is partially mediated by
BMI’s effect on oxidative stress but not inflammation or antifibrinolysis. They found increases
in BMI of 5 kg/m2 to be independently associated with 26.5% increases in the odds of
postoperative AKI.
Salim S. et al. (2009) conducted a retrospective cohort analysis of 17,630 consecutive patients
who underwent surgical procedures (CABG, valve surgery, or both) at St. Luke's Episcopal
Hospital. They found that obesity was associated with a significant increase in postoperative
renal insufficiency in patients undergoing cardiac surgeries.
This effect seen in the entire cohort, as well as in patients undergoing isolated CABG or isolated
valve surgeries—was attributable mostly to an increase in postoperative renal failure that did
not require dialysis. The risk was graded with the increasing risk that is associated with an
increase in the severity of obesity. The development of postoperative renal insufficiency was
associated with a significantly higher mortality rate and with a longer length of stay.
Obesity is associated with oxidative stress and endothelial dysfunction. Obese patients are at a
higher risk of developing hypertension and diabetes, which are also associated with elevated
inflammatory response and impaired endothelial function. The use of cardiopulmonary bypass
for cardiac surgeries has been shown to be associated with an up-regulation of the inflammatory
12
cascade. This, together with the prevalence of risk factors associated with the development of
postoperative renal insufficiency and the risk associated with obesity itself, means that obese
patients may have a higher incidence of postoperative renal insufficiency.
Their showed that the presence of even mild forms of obesity (a BMI of 30 to 34.99 kg/m 2)
was independently associated with the occurrence of postoperative renal insufficiency and that
patient cohorts with higher BMIs have higher incidences of postoperative renal insufficiency—
which suggests a dose-response relationship. Most of the postoperative renal failure
attributable to obesity did not require dialysis, but it was associated with a significantly longer
hospital stay. Their findings extend the findings from earlier studies to show that obesity is
associated also with an increased risk of renal insufficiency in subgroups that include patients
undergoing isolated CABG or isolated valve surgeries. These subgroups have not been well
studied before.
Katie E. et al. (2015) study confirmed that obesity was independently associated with an
increased incidence of AKI after cardiac surgery. Obesity is a state of chronic inflammation.
Central obesity is known to be associated with chronic kidney disease (CKD) independently
of other components of the metabolic syndrome.
Obesity is also associated with inflammation, and the presence of inflammation modifies the
associations of central obesity and CKD. Obesity itself is known to lead to CKD via its
association with other risks, such as hypertension, diabetes, and atherosclerosis; however, more
recently it has become widely accepted as an independent risk factor itself.
Examination of other etiologic factors to date has focused on the proinflammatory properties
of adipose tissue ad release of leptin, adiponectin, tumor necrosis factor-α, interleukin-6 and
10, monocyte chemoattractant protein-1, and resistin.
Their study concluded that, independently of known risk factors (age, anemia,
gender, hyperlipidemia,
smoking
history, cerebrovascular
13
disease,
diabetes, blood
transfusion, cardiopulmonary bypass duration, and postoperative blood product transfusion
and hematocrit), obesity with BMI 30 kg/m2 or greater is associated with an increased risk
of AKI after cardiac surgery.
In the study of Avinash B. et al. (2014), BMI ≥ 40 kg/m2 (obesity class III) was associated
with an increased risk of developing AKICPB. Class III obese patients were almost four times
more likely to develop AKICPB when compared with patients in lower BMI classes. The
authors cannot fully explain the disproportionate risk in the relationship between BMI 4 40
kg/m2 and increasing risk of developing AKICPB. Even though the number of patients in the
morbidly obese cohort was small, the magnitude of risk of developing AKICPB after adjusting
for other covariates was significant.
The links between obesity and development of chronic kidney disease have been known for
several years, but the association with acute kidney injury is less clear. Visceral adipose tissue,
through secreted hormones and cytokines, (including leptin, adiponectin, tumor necrosis
factor-α, angiotensinogen, interleukin-6, and C-reactive protein) coupled with insulin
resistance and hyperinsulinemia in obese patients, can lead to inappropriate activation of the
renin-angiotensinaldosterone axis and increased oxidative stress in the kidneys.
Obese patients have elevations of both kidney plasma flow and glomerular filtration rate that
exceed those of controls by 31% and 51%, respectively, thereby promoting glomerular
capillary hypertension3 In addition, there is a higher prevalence of glomerulomegaly, focal
segmental glomerulosclerosis, mesangial sclerosis, mesangial hypercellularity, and podocyte
hypertrophy in patients with BMI 440 kg/m2 even with normal kidney function.
Thus, in morbidly obese patients, it is likely that the degree of elevation of BMI per se may
increase the risk of developing glomerular lesions. The inflammatory and endocrine factors
associated with visceral obesity, underlying occult and declared structural changes in kidneys
14
of obese patients, and the pathophysiologic contribution of CPB may cumulatively account for
the increased risk of the AKICPB in the morbidly obese patient.
Kerstin A. et al. (2013) Both obesity and the metabolic syndrome have been identified as
powerful predictors of CKD and ESRD. The abnormalities of renal structure in obese and
morbidly obese individuals include increased kidney weight, glomerulomegaly, disorder of
podocytes, mesangial expansion, and, more recently, also abnormalities of the renal
interstitium (ie, tubular atrophy and interstitial fibrosis), and accompanying vascular
alterations. This is accompanied by functional abnormalities such as renal hyperperfusion,
increased filtration fraction, albuminuria, or proteinuria.
The link between progressive kidney disease, diabetic nephropathy, and visceral obesity or the
metabolic syndrome is of enormous public health importance. Apart from aggravating most
primary kidney diseases, obesity was shown to cause a specific renal disease with uncertain
prognosis (ie, ob-FSGS). In addition, obesity and the closely related metabolic syndrome
predispose to diabetes mellitus type 2 and diabetic nephropathy, which is the leading cause of
endstage renal failure in the world.
It is of major clinical importance, however, that at least in obesity an improvement of renal
symptoms could be achieved by loss of body weight, for example, by lifestyle modification or
perhaps more importantly by bariatric surgery. Thus, prevention and treatment of the common
phenomenon of obesity may reduce CKD incidence in the general population.
This study shows that both GFR and RPF of extremely obese patients are increased, the GFR
being relatively more elevated than the RPF, resulting in an increased filtration fraction. The
augmented RPF suggests a state of renal vasodilatation involving, mainly or solely, the afferent
arteriole. RPF is a determinant of GFR independently of the capillary hydrostatic pressure: its
increase is predicted to lower the intraluminal concentration of macromolecules as blood flows
axially along the glomerular capillaries. This results in a decrease in glomerular intracapillary
15
oncotic pressure, thus enhancing the net ultrafiltration pressure and contributing to the elevated
GFR.
However, because the increase in filtration fraction offsets the effect of the increase in RPF on
the glomerular oncotic pressure, factors other than oncotic pressure must have contributed to
the elevation of GFR.
Although within the normal range, the MAP was higher in the obese group than in the control
group. The combination of increased arterial pressure abnormally transmitted to the glomerular
capillaries through a dilated afferent arteriole is expected to cause an elevated glomerular
capillary pressure, resulting in an increased transcapillary pressure gradient ΔP and an elevated
GFR.
Obesity is associated with marked insulin resistance. The group of obese patients studied here
exhibited features suggestive of marked insulin resistance, which was correlated with RPF,
GFR, and filtration fraction.
In summary, the elevated GFR of very obese nondiabetic patients is associated with an
increased RPF. The analysis of dextran-sieving data suggests, but does not prove that the
pathogenesis of hyperfiltration differs from that of the diabetic kidney, in that it is mainly or
solely due to an increased ΔP. The role of insulin resistance as a factor contributing to these
glomerular hemodynamics changes remains to be clarified.
Kristian K. et al. (2014) The authors present a study conducted on an up-to-date cohort, who
underwent cardiac surgery during 2012, in which the majority of patients received ibuprofen
and gentamicin. The principal finding of the study was a high incidence of AKI after isolated
CABG or CABG in combination with valve surgery. The majority of patients had the lowest
stage AKI according to AKIN criteria. However, even AKIN stage 1 was associated with a
marked increase in mortality compared to patients without AKIN. AKIN stage 1 was present
in 23% of the patients. Possible contributors to the high incidence of AKI in the authors’ center
16
could be the routine use of gentamicin and ibuprofen. Gentamicin is known to be nephrotoxic,
and ibuprofen generally is considered contraindicated in CABG patients. The U.S. Food and
Drug Administration has issued a warning on ibuprofen use in CABG patients. This is on the
basis of its possible thrombogenic properties and not nephrotoxicity. The rationale behind the
possible nephrotoxicity of ibuprofen is its inhibition of the synthesis of prostaglandins that
mediate dilation of the afferent arteriole in the glomerulus of the kidney in situations with
decreased perfusion pressure. No randomized controlled studies have been published looking
at ibuprofen and renal function in cardiac surgery patients. Two large studies have shown an
increased cardiovascular risk using ibuprofen in general. However, nothing yet has been
concluded about the risk of using ibuprofen for shorter periods of time.
Furthermore, the authors found that the relationship between AKI and mortality was
statistically independent, taking multiple perioperative confounders into account. This
indicated that AKI still continues to be an important cause of mortality regardless of the
perioperative risk profile among patients undergoing cardiac surgery.
The authors found a high incidence of AKI in a contemporary cohort of cardiac surgical
patients, the majority of whom received ibuprofen and gentamicin. AKI was associated
independently with a higher mortality even in AKIN stage 1 regardless of the perioperative
risk evaluation.
Luis F. et al. (2008) indicated that CKD is associated with inflammation and oxidative stress,
and this metabolic milieu may contribute significantly to the excessive cardiovascular disease
seen in these patients. In the control group, BMI and body fat percentage were also associated
with the measured markers, highlighting the contribution of adiposity to the metabolic milieu
regardless of kidney function.
17
Inflammation and oxidative stress are known to be prevalent in patients with CKD. We
previously showed that increased markers of oxidative stress and inflammation are present in
stages 3 to 5 CKD before initiation of maintenance dialysis but that levels did not correlate
with eGFR.
These findings suggest that the mere presence of CKD, regardless of the level of GFR, is an
important driver of the inflammatory and oxidative process. Similarly, adiposity is highly
correlated with inflammation and oxidative stress. Visceral fat secretes proinflammatory
cytokines that attract macrophages to infiltrate adipocytes, leading to further release of
cytokines and oxygen free radicals, which may ultimately cause oxidative damage and
atherosclerosis. Our findings suggest that adiposity may be a potent, independent amplifier to
the inflammatory and oxidative milieu already present in CKD.
These findings suggest that obesity, via vascular damage induced by oxidative stress and
inflammation, may be an additional nontraditional cardiac risk factor responsible for the
accelerated atherosclerosis and high cardiovascular mortality in the earlier stages of CKD. The
potential contribution of the inflammatory and oxidative state to atherogenesis is clinically
significant when considering that the majority of patients with CKD, approximately 19 million
in the United States alone, will succumb to atherosclerotic disease before requiring renal
replacement therapy.
In addition to contributing to cardiovascular risk, obesity is an independent predictor of
incident CKD and progression to ESRD. Thus, it is logical to hypothesize that weight loss may
be an effective therapeutic strategy that not only prevents the development of CKD but also
mitigates the inflammatory and oxidative burden that may lead to accelerated atherosclerosis
in renal disease.
According to Robbert et al. (2005), surprisingly little is known about the potential impact of
body size at the time of CABG on intermediate to longer term survival. Robert H. et al. (2004)
18
analysis showed that 12-year CABG survival was significantly worse for very small and very
obese. For very small, the increased death hazard was evident early during year 1 and late after
year 8. In contrast, excluding early deaths increases the very obese adjusted RR from 1.44 to
1.65.
Moreover, very obese showed particularly greater death hazard between 1 and 6 years. It is not
obvious why very small and very obese do worse than normal in the long-term even after
multivariate adjustment. Follow-up data after discharge were limited to patient death only. Yet,
one can speculate about possible reasons for these results. First, the two extreme size groups
are associated with a greater propensity of post-CABG complications affecting a number of
vital organs. Such injury (eg, renal failure) may have sustained long-term effects on organ
function and hence on survival.
Second, small patients are susceptible to increased CPB hemodilutional anemia, and
subsequently require more transfusions. Increased hemodilutional anemia is associated with
vital organ injury with possible long-term effects. Both excessive hemodilution and transfusion
are linked to worse long-term mortality.
Fortunately, these risks in small patients are potentially modifiable by changes in CPB practice
so that excessive hemodilution and transfusions are minimized, for example, use of smaller
bypass circuits, retrograde autologous priming, or off-pump surgery. Third, compared with
comorbidity-matched normal patients, very small and very obese may be associated with an
increased likelihood for developing new comorbidities or exacerbation of already existing
comorbidities. For example, very obese are probably more likely to become diabetic or develop
hypertension at a given interval after CABG.
We did not find appreciable differences in survival and death hazard for the slightly small and
moderately obese groups when compared with matched normal patients. This is in contrast to
19
two recent population studies that indicated that even mild obesity is associated with increased
years of life lost.
The development of postoperative renal insufficiency is an important clinical event, because it
is associated with an increase in death and with prolonged stays in the intensive care unit after
cardiac surgery.
The aim of this study was to determine whether obesity is an independent predictor of
postoperative renal insufficiency in patients undergoing cardiac surgeries, including patients
undergoing isolated coronary artery bypass grafting (CABG), isolated valve surgeries, and
combined CABG and valve surgeries. We also investigated the possible association between
obesity and increased severity of postoperative renal failure (that is, postoperative renal failure
requiring in-hospital dialysis)
20
3.0: METHODS
3.1: Objective of Study
Prior studies have not demonstrated a consistent association between BMI and AKI after
cardiac surgery. This study is aimed to study the effect of BMI of normal weight and
overweight patients on AKI after cardiopulmonary bypass.
3.2: Hypothesis
It was hypothesized that extreme weight has a worsening effect on the post-operative AKI of
patients undergoing cardiopulmonary bypass.
3.3: Study Design
Data were entered prospectively into the Patient Analysis & Tracking System. The research
question was posted after the collection of the data but before any data analysis. The study was
approved by the Punjab Institute of cardiology board and complies with the Strengthening the
Reporting of Observational Studies in Epidemiology reporting requirements for observational
studies.
Renal complication: It included post-operative creatinine 200 mol/l and acute renal failure as
defined by the requirement of hemodialysis.
Body Mass Index: BMI was defined as the weight in kilograms divided by the square of
the height in meters. Our study only included the overweight and normal weight patients
undergoing cardiac surgery.
normal weight (BMI 18.5–<25 kg/m2)
overweight (BMI 25–<30 kg/m2)
3.4: Study Duration
21
We performed a prospective, observational cross-sectional study encompassing all adult
cardiac surgical procedures performed in the Punjab Institute of Cardiology, Lahore between
April 1, 2021, and Sepember 30, 2021.
3.5: Sampling Technique
The variables used for our analysis included age, sex, history of hypertension, diabetes mellitus,
preoperative renal insufficiency, hyperlipidemia, low left ventricular ejection fraction (defined
as <0.35), need for urgent or repeat surgery, need for an intra-aortic balloon pump, New York
Heart Association class at the time of surgery, and aortic cross-clamp time.
Standard data are collected prospectively for all patients undergoing CABG and valve surgery
at our institution.
The data collection form includes five sections that are filled in consecutively by
anesthetist, surgeon, intensive care unit (ICU), high dependency unit (HDU), and ward
nurses.
Cardiac catheterization was performed using standard methods during the course of routine
clinical care.
Angiography reports were reviewed before surgery to assess the severity of coronary artery
disease, expressed as the number of diseased vessels.
Priority of surgery was assessed by the cardiothoracic surgeon and was defined as follows:
1. Emergency (the surgery should be performed within hours to prevent morbidity or
death)
2. Urgent (medical factors require the patient to stay in hospital waiting for an operation)
3. Elective (the clinical status of the patient allows discharge from hospital with
readmission for surgery at a later date).
3.6: Surgical technique and postoperative management.
22
Anesthetic and surgical techniques were standardized for all patients and have been
previously reported.
At the end of the surgery, patients were transferred to the ICU and were extubated as soon
as they met the following criteria:
Hemodynamic stability
No excessive bleeding (80 ml/h)
Normothermia,
Consciousness
Pain control.
Postoperatively, fluid management and electrolyte deficiency were managed as previously
reported.
The patient's BMI assessed the extent of obesity in our study. BMI, derived from Quetelet's
formula, is calculated by the weight in kilograms divided by the square of the height in
meters [14,15].*
Different techniques of coronary revascularization were used in our study. These included
operations done with and without CPB.
The technique of myocardial protection for patients done on CPB again varied according
to operator preference. Although blood cardioplegia was the favoured choice, cold
crystalloid cardioplegia was also used by some surgeons. Mammary arteries were harvested
as a pedicle.
3.7: Inclusion Criteria:
For each operation, data were recorded on patient characteristics and demographics,
comorbidities, intraoperative factors, and postoperative out-comes. Administrative data were
also extracted.
23
The analysis data set was obtained by including all cases with complete data on a set
of key preoperative, intraoperative, and post-operative variables as follows: age, BMI, sex,
left ventricular ejection fraction category, history of myocardial infarction, renal
impairment, diabetes mellitus on medication, previous cardiac surgery, operation type, and
cardiopulmonary bypass use.
3.8: Exclusion Criteria:
We excluded confused states, transient events and intellectual impairment from our study to
avoid any subjective bias. Patients undergoing salvage surgical procedures (cardiac arrest
before induction), patients with critical preopera-tive state (ventilated, cardiogenic shock,
inotropic support, intra-aortic balloon pump), and patients with stage 5 chronic kidney disease
(dialysis) were excluded. Patients for whom it was not possible to calculate the BMI or for
whom the sex of the patient, operation type, or discharge status was missing were also
excluded.
Pre-operative data were collected during the patient's admission as part of routine clinical
practice on the following variables: age, sex, BMI, urgency of operation, prior cardiac
surgery, New York Heart Association (NYHA) functional class, history of myocardial
infarction, smoking, diabetes, hypercholesterolaemia, hypertension, peripheral vascular
disease, cerebrovascular disease, respiratory disease, renal dysfunction, intravenous
nitroglycerin therapy, cardiogenic shock, and intra-aortic balloon pump support as well as
the extent of coronary disease, and left ventricular ejection fraction.
Perioperative data were collected on the use of cardiopulmonary bypass (CPB) and the
number and type of grafts were also collected.
The Post-operative data for this study were in-hospital mortality, re-exploration for
bleeding, renal failure, duration of mechanical ventilation and post-operative length of stay.
3.9: Data Collection
24
Data were prospectively collected on a total of 103 consecutive patients undergoing isolated
CABG surgery or valve surgery. Data collection methods and definitions have been described
in detail previously
These data are collected prospectively and undergo robust validation and checking
procedures to maintain data quality.
Duplicate records and nonadult cardiac surgery entries were removed
Transcriptional discrepancies were harmonized
Clinical and temporal conflicts and extreme values were corrected or removed
No attempt to replace missing values was made
The need to obtain informed consent was waived because patients’ identifiable information
was either removed or pseudonymized.
3.10: Statistical Analysis
This research was conducted using open epic software. Version 20.00 of the Social Science
Statistical Package was used for the statistical analysis. The data of categorical variables were
presented as counts and percentages. Descriptive frequencies were used for analyzing all
categorical data.
25
4.0: RESULTS
Table No 4: Frequency Distribution with Respect to age:
AGE OF PATIENTS
Mean Standard Deviation
52.23 12
Minimum Age
12 years
Maximum Age
75 years
Out of 107 patients, their age ranges from as young as 12 years old to as elder as 75 years old
patients. The mean age of the patients was 52 12 years (52 years with a standard deviation
of 12 years).
26
Graph No 1: Graphical Presentation with respect to Age Stratification
The age group 1 has a frequency of 3 which is 2.8% of the total age of patients.
The age group 2 (10 – 20 years) has a frequency of 5 (21 – 30 years) which is 4.7% of the total
age of under observation patients.
The age group 3 (31 – 40 years) has a frequency of 8 which is 7.5% of the total age of under
observation patients.
The age group 4 (41 – 50 years) has a frequency of 25 which is 23.5% of the total age of under
observation patients.
The age group 5 (51 – 60 years) has a frequency of 39 which is 36.4% of the total age of under
observation patients.
27
The age group 6 (61 – 70 years) has a frequency of 23 which is 21.5% of the total age of under
observation patients.
The age group 7 (71 – 80 years) has a frequency of 4 which is 3.7% of the total age of under
observation patients.
As we can see most of our patients were ranging from age 51 years to 60 years. While the
minimum age group frequency was in group 1 of age 10 – 20 years.
28
Graph No 2: Pei Chart Presentation with respect to Gender
In 107 samples under observation, 76 of them were males (71.0% of the sample size), while
31 of them were females (i.e. 29% of the sample size).
29
Table no# 5: Distribution of Age in normal weight and overweight groups of BMI
Groups according to BMI
Age of patient
(Years)
Mean S.D
50.72 15
Frequency
46
Minimum Age
17
Maximum Age
75
Mean S.D
53.38 10
Over weight
Frequency
61
(BMI 25-30)
Minimum Age
12
Maximum Age
69
Normal weight
(BMI 18.5-24)
In this table the age distribution across the two groups of normal weight BMI and overweight
BMI has been shown. The normal weight BMI group has patients with a mean age of 50.72
15 (Mean Standard deviation) years. The minimum age of the patient in this group is of 17
years. However, the maximum age of 75 years has been recorded in this group. Total 46
patients were present in this group
The overweight BMI group has 61 total patients. Among this group patients has a mean age of
53.38 10 (Mean Standard deviation) years. The minimum aged patient in this group is of
12 years. However, the maximum age of 69 years has been recorded in this group.
30
Table no# 6: Distribution of Risk Factors in normal weight and overweight groups of
BMI
Groups according to BMI
Risk Factors
Normal weight (BMI 18.524)
Gender
Surgery type
Smoking
Hypertension
Diabetes Mellitus
Asthma
CKD
Dyslipidemia
Over weight (BMI 25-30)
Frequency
%
Frequency
%
Male
32
69.6%
44
72.1%
Female
14
30.4%
17
27.9%
Isolated CABG
34
73.9%
52
85.2%
Isolated Valve
12
26.1%
8
13.1%
CABG + Valve
0
.0%
1
1.6%
yes
12
26.1%
17
27.9%
no
34
73.9%
44
72.1%
yes
14
30.4%
36
59.0%
no
32
69.6%
25
41.0%
yes
12
26.1%
26
42.6%
no
34
73.9%
35
57.4%
yes
0
.0%
1
1.6%
no
46
100.0%
60
98.4%
yes
0
.0%
0
.0%
no
46
100.0%
61
100.0%
yes
0
.0%
0
.0%
no
46
100.0%
61
100.0%
31
Gender: The normal weight BMI group has 32 male patients, which is 69.6 % of the member
of this group. It has frequency of 14 female patients, which is 30.4 % of the member of this
group. The overweight BMI group has 44 male patients, which is 72.1 % of the member of this
group. It has frequency of 17 female patients, which is 27.9 % of the member of this group.
Type of surgery: In normal weight BMI, group 34 patients (73.9 %) went isolated CABG
(Coronary artery bypass graft) surgery, 12 patients (26.1 %) underwent isolated valve surgery,
and no patient had CABG + valve surgery. In overweight BMI group, 52 patients (85.2 %)
went isolated CABG surgery, 8 patients (13.1 %) underwent isolated valve surgery, and 1
patient (1.6 %) had CABG + valve surgery.
Smoking: In normal weight BMI group, 12 patients (26.1 %) were active smokers, while 34
patients (73.9 %) were not active smoker. In overweight BMI group, 17 patients (27.9 %) were
active smokers, while 44 patients (72.1 %) were not active smoker.
Hypertension: In normal weight BMI group, 14 patients (30.4 %) were hypertensive patients,
while 32 patients (69.6 %) were not hypertensive. In overweight BMI group, 36 patients (59
%) were hypertensive patients, while 25 patients (41 %) were not hypertensive.
Diabetes Mellitus: In normal weight BMI group, 12 patients (26.1 %) were diabetic patients,
while 34 patients (73.9 %) were not diabetic. In overweight BMI group, 26 patients (42.6 %)
were diabetic patients, while 35 patients (57.4 %) were not diabetic.
Asthma: In normal weight BMI group, no patient had asthma, while 46 patients (100 %) had
not contracted asthma. In overweight BMI group, 1 patient (1.6 %) had asthma, while 60
patients (98.4 %) had not contracted asthma.
Chronic Kidney Disease (CKD): In both normal and overweight BMI group, no patient had
chronic kidney disease.
Dyslipidemia: In both normal and overweight BMI group, no patient had dyslipidemia.
32
Table no# 7.1: Distribution of prognostic pre-operative variables in normal weight and
overweight groups of BMI
Groups according to BMI
Pre-operative variables
Normal weight (BMI
Over weight (BMI 25-
18.5-24)
30)
Frequency
%
Frequency
%
I
1
2.2%
1
1.6%
II
20
43.5%
19
31.1%
III
20
43.5%
40
65.6%
IV
5
10.9%
1
1.6%
Pre-operative
yes
24
52.2%
25
41.0%
Diuretics Use
no
22
47.8%
36
59.0%
NYHA class
NYHA class: In normal weight BMI group, 1 patient (2.2 %) had NYHA class I, 20 patients
(43.5 %) had NYHA class II, 20 patient (43.5 %) had NYHA class III, 5 patient (10.9 %) had
NYHA class IV. In overweight BMI group, 1 patient (1.6 %) had NYHA class I, 19 patients
(31.1 %) had NYHA class II, 40 patient (65.6 %) had NYHA class III, 1 patient (1.6 %) had
NYHA class IV.
Pre-operative diuretics use: In normal weight BMI group, 24 patients (52.2 %) use diuretics
before surgery, while 24 patients (47.8 %) were not taking diuretics before medicine. In
overweight BMI group, 25 patients (41 %) use diuretics before surgery, while 36 patients (59
%) were not taking diuretics before medicine.
33
Table no# 7.2: Distribution of prognostic pre-operative variables in normal weight and
overweight groups of BMI
Groups according to BMI
Normal weight (BMI 18.5-24)
Over weight (BMI 25-30)
Pre-operative
variables
Maximum Minimum
Maximum Minimum
Mean S.D
Mean S.D
Value
Value
Value
Value
Ejection fraction
66.00
20.00
70.00
25.00
62.57 91
53.61 10
%
Pre-Operative Hb
19.10
10.30
16.50
7.50
13.43 2
13.13 2
%
Pre-operative Urea
60.00
18.00
75.00
10.0
32.76 12
33.30 16
mg/dl
Pre-operative
Creatinine
1.40
.40
2.20
0.40
0.87 0.27
0.93 0.3
mg/dl
Pre-operative Urine
output
.00
1000.00
0.00
227.65 196 1000.00
223.85 244
mL
Ejection fraction: In normal weight BMI group, the mean ejection fraction was 62.57 91,
with maximum value of 66% and minimum value of 20% ejection fraction. In overweight
BMI group, the mean ejection fraction was 53.61 10, with maximum value of 70% and
minimum value of 25% ejection fraction.
Pre-Operative Hb: In normal weight BMI group, the mean Pre-Operative Hb was 13.43 2,
with maximum value of 19% and minimum value of 10% Pre-Operative Hb. In overweight
BMI group, the mean Pre-Operative Hb was 13.13 2, with maximum value of 16% and
minimum value of 7.5% Pre-Operative Hb.
Pre-Operative Urea: In normal weight BMI group, the mean pre-operative urea was 32.76
12, with maximum value of 60 mg/dl and minimum value of 18 mg/dl pre-operative urea.
In overweight BMI group, the mean pre-operative urea was 33.30 16, with maximum value
of 75 mg/dl and minimum value of 10 mg/dl pre-operative urea.
34
Pre-Operative Creatinine: In normal weight BMI group, the mean pre-operative creatinine
was 0.87 0.27, with maximum value of 1.4 mg/dl and minimum value of 0.4 mg/dl preoperative creatinine. In overweight BMI group, the mean pre-operative creatinine was 0.93
0.3, with maximum value of 2.2 mg/dl and minimum value of 0.4 mg/dl pre-operative
creatinine.
Pre-Operative Urine Output: In normal weight BMI group, the mean pre-operative urine
output was 227.65 196, with maximum value of 1000 ml and minimum value of no preoperative urine output recorded. In overweight BMI group, the mean pre-operative urine
output was 223.85 244, with maximum value of 1000 ml and minimum value of 0 ml preoperative urine output.
35
Statistical distribution of prognostic intra-operative variables with respect to BMI
based groups:
Table no# 8: Distribution of prognostic intra-operative variables in normal weight and
overweight groups of BMI
Groups according to BMI
Intra-operative
variables
Normal weight (BMI 18.5-24)
Mean
S.D
Maximum Minimum
Over weight (BMI 25-30)
Mean
S.D
Maximum Minimum
Bypass time
(Minutes)
115.70
43
227.00
57.00
121.18
50
320.00
35.00
Cross clamp time
(Minutes)
61.54
34
180.00
.00
60.69
31
174.00
.00
Bypass time: In normal weight BMI group, the mean bypass time was 115.70 43, with
maximum value of 227 minutes and minimum value of 57 minutes of bypass time. In
overweight BMI group, the mean bypass time was 121.18 50, with maximum value of 320
minutes and minimum value of 35 minutes of bypass time.
Cross-Clamp time: In normal weight BMI group, the mean cross-clamp time was 61.54
34, with maximum value of 180 minutes and minimum value of 0 minutes of cross-clamp
time. In overweight BMI group, the mean cross-clamp time was 60.69 31, with maximum
value of 320 minutes and minimum value of 0 minutes of cross-clamp time.
36
Statistical distribution of prognostic post-operative variables with respect to BMI based
groups:
Table no# 9.1: Distribution of prognostic post-operative variables in normal
weight and overweight groups of BMI
Groups according to BMI
Normal weight (BMI
Over weight (BMI
18.5-24)
25-30)
Post-operative variables
Frequency
%
Frequency
%
yes
1
2.2%
0
0.0%
no
45
97.8%
61
Dialysis
100.0
%
Post-operative
yes
31
67.4%
46
75.4%
Diuretics Use
no
15
32.6%
15
24.6%
Blood units used
46
61
Dialysis: In normal weight BMI group, only 1 patients (2.2 %) went on dialysis, while 45
patients (97.8 %) did not required dialysis. In overweight BMI group, no patient required
dialysis treatment.
Post-operative diuretics use: In normal weight BMI group, 31 patients (67.4 %) use diuretics
after surgery, while 15 patients (32.6 %) were not taking diuretics. In overweight BMI group,
46 patients (75.4 %) use diuretics after surgery, while 15 patients (24.6 %) were not taking
diuretics after surgery.
Blood Units: For normal weight BMI group 46 units of blood were used for patients, while for
overweight BMI group, 61 units of blood were used.
37
Statistical distribution of prognostic post-operative variables with respect to BMI based
groups:
Table no# 9.2: Distribution of prognostic post-operative variables in normal weight and
overweight groups of BMI
Groups according to BMI
Post-operative
Normal weight (BMI 18.5-24)
Over weight (BMI 25-30)
variables
Mean S.D Maximum Minimum Mean S.D Maximum Minimum
Post-operative
Hb
11.05 1
13.70
8.00
10.96 1.4
14.70
7.90
%
Post-operative
Urea
mg/dl
Post-operative
Creatinine
mg/dl
Post-operative
Urine output
mL
47.48 40
174.00
10.0
45.08 36
255.00
14.00
1.21 0.4
3.90
.40
1.17 0.5
2.60
.40
2837.39
1132
4800.00
200
3091.41
847
5020.00
990.00
Post-Operative Hb: In normal weight BMI group, the mean post-Operative Hb was 11.05
1, with maximum value of 13.7% and minimum value of 8% post-Operative Hb. In
overweight BMI group, the mean post-Operative Hb was 10.96 1.4, with maximum value
of 14.7% and minimum value of 8% post-Operative Hb.
Post-Operative Urea: In normal weight BMI group, the mean post-operative urea was 47.48
40, with maximum value of 174 mg/dl and minimum value of 10 mg/dl post-operative urea.
In overweight BMI group, the mean post-operative urea was 45.08 36, with maximum value
of 255 mg/dl and minimum value of 14 mg/dl post-operative urea.
Post-Operative Creatinine: In normal weight BMI group, the mean post-operative
creatinine was 1.21 0.4, with maximum value of 3.9 mg/dl and minimum value of 0.4 mg/dl
post-operative creatinine. In overweight BMI group, the mean post-operative creatinine was
38
0.93 0.3, with maximum value of 2.6 mg/dl and minimum value of 0.4 mg/dl post-operative
creatinine.
Post-Operative Urine Output: In normal weight BMI group, the mean post-operative urine
output was 2837.39 1132, with maximum value of 4800 ml and minimum value of 200 ml
post-operative urine output recorded. In overweight BMI group, the mean post-operative urine
output was 3091.41 847, with maximum value of 5020 ml and minimum value of 990 ml
post-operative urine output.
Table no# 10: Distribution of clinical outcome in normal weight and overweight groups
of BMI
Groups according to BMI
Normal weight (BMI
Over weight (BMI
18.5-24)
25-30)
Clinical Outcome
Frequency
%
Frequency
%
Acute Kidney
yes
6
13.0%
7
11.5%
Injury
no
40
87.0%
54
88.5%
Acute Kidney Injury: In normal weight BMI group, 6 patients (13 %) had acute kidney
injury, while 40 patients (87 %) were not suffering from acute kidney injury. In overweight
BMI group, 7 patients (11.5 %) had acute kidney injury, while 54 patients (88.5 %) were not
suffering from acute kidney injury.
39
5.0 Discussion:
In our study the normal weight BMI group has 32 male patients, which is 69.6 % of the member
of this group. It has frequency of 14 female patients, which is 30.4 % of the member of this
group. The overweight BMI group has 44 male patients, which is 72.1 % of the member of this
group. It has frequency of 17 female patients, which is 27.9 % of the member of this group.
While comparing the study of Kristy et al. (2014) Health related Quality of Life was associated
with gender and age, but not weight status or socio-economic status; with males and younger
adolescents having higher HRQoL scores than their female and older adolescent counterparts
(both p < 0.05). There was also a significant interaction of weight status by gender whereby
overweight females had poorer HRQoL (-.06 units) relative to healthy weight females
(p < 0.05).
In our study the normal weight BMI group, 12 patients (26.1 %) were active smokers, while
34 patients (73.9 %) were not active smoker. In overweight BMI group, 17 patients (27.9 %)
were active smokers, while 44 patients (72.1 %) were not active smoker.
While comparing the study of Tian J. et al. (2015), individuals who quit smoking gained, on
average, approximately 4.1 kg or 1.1 kg m−2 BMI units over about 5 years. The MD in weight
gain between quitters and continuing smokers was 2.6 kg or 0.6 kg m−2 BMI units. Because
of the well-documented health benefits of quitting smoking, clinicians should inform smokers
about the likelihood of weight gain and encourage them to maintain or adopt a healthy lifestyle
to avoid excess weight gain, such as engaging in moderate physical activity. Better designed
observational studies and smaller well-controlled clinical trials are needed to determine what
is associated with greater weight gain in quitters than continuing smokers.
40
In our study the normal weight BMI group, 14 patients (30.4 %) were hypertensive patients,
while 32 patients (69.6 %) were not hypertensive. In overweight BMI group, 36 patients (59
%) were hypertensive patients, while 25 patients (41 %) were not hypertensive.
While comparing the study of CHIANG, B. et al. (1969), weight reduction has been shown to
lower blood pressure, and it may bring about a more favorable prognosis in obese hypertensive
persons. Possible mechanisms that may be responsible for the frequent association between
obesity and hypertension have been discussed. Irrespective of the underlying pathophysiologic
mechanisms, the adverse metabolic and hemodynamic effects of obesity upon hypertension
impose an extra burden and strain on the circulatory system and compromise its functional
adequacy. Although it is not precisely known to what extent weight reduction alone may be
effective in controlling or preventing the lesser degrees of hypertension, the control of obesity
should be an intrinsic part of any therapeutic or preventive antihypertensive regimen.
In our study the normal weight BMI group, 12 patients (26.1 %) were diabetic patients, while
34 patients (73.9 %) were not diabetic. In overweight BMI group, 26 patients (42.6 %) were
diabetic patients, while 35 patients (57.4 %) were not diabetic.
While comparing the study of Mercedes R. et al. (2012), The proportion of adults who were
normal weight at the time of incident diabetes ranged from 9% to 21% (overall 12%). During
follow-up, 449 participants died: 178 from cardiovascular causes and 253 from
noncardiovascular causes (18 were not classified). The rates of total, cardiovascular, and
noncardiovascular mortality were higher in normal-weight participants (284.8, 99.8, and 198.1
per 10 000 person-years, respectively) than in overweight/obese participants (152.1, 67.8, and
87.9 per 10 000 person-years, respectively). After adjustment for demographic characteristics
and blood pressure, lipid levels, waist circumference, and smoking status, hazard ratios
comparing normal-weight participants with overweight/obese participants for total,
cardiovascular, and noncardiovascular mortality were 2.08 (95% CI, 1.52-2.85), 1.52 (95% CI,
41
0.89-2.58), and 2.32 (95% CI, 1.55-3.48), respectively. Adults who were normal weight at the
time of incident diabetes had higher mortality than adults who are overweight or obese.
In our study the normal weight BMI group, no patient had asthma, while 46 patients (100 %)
had not contracted asthma. In overweight BMI group, 1 patient (1.6 %) had asthma, while 60
patients (98.4 %) had not contracted asthma. While comparing the study of Fernendo et al.
(2011), The study population consisted of 1049 subjects, and the median age for asthma onset
was 10 years (interquartile range, 4-25 years); 48% had late-onset asthma (≥12 years of age),
and 52% had early-onset asthma (<12 years of age). Compared with obese subjects with lateonset asthma, obese subjects with early-onset asthma had more airway obstruction, bronchial
hyperresponsiveness, and higher odds ratios of ever having 3 or more previous oral steroid
tapers per year or intensive care unit admissions for asthma per preceding year (interactions
between obesity and age of asthma onset were P = .055 and P = .02, respectively). In subjects
with early-onset asthma but not in subjects with late-onset asthma, there was a significant
association between increasing BMI and duration of asthma after adjusting for confounders.
The interaction between asthma duration and age of asthma onset was a P value of less than
.01.
Asthmatic subjects are differentially affected by obesity based on whether they had asthma
early (<12 years of age) or later in life. These results highlight the need to understand obesity
as a comorbidity that affects specific clinical phenotypes and not all asthma subjects alike.
In normal weight BMI group, 1 patient (2.2 %) had NYHA class I, 20 patients (43.5 %) had
NYHA class II, 20 patient (43.5 %) had NYHA class III, 5 patient (10.9 %) had NYHA class
IV. In overweight BMI group, 1 patient (1.6 %) had NYHA class I, 19 patients (31.1 %) had
NYHA class II, 40 patient (65.6 %) had NYHA class III, 1 patient (1.6 %) had NYHA class
IV.
42
While comparing the study of Wuhua J. et al. (2016), preoperative NYHA scores >2, previous
cardiac surgery, and postoperative LCOS were also found to be risk factors for CSA‐AKI in
our study. Among the above factors, preoperative NYHA scores >2 and previous cardiac
surgery are not modifiable. The LCOS may result from delayed recovery of post‐op cardiac
function and cause pulmonary edema and pulmonary infection, which in turn aggravate LCOS
in a vicious circle.30 For those CSA‐AKI patients who were unable to eliminate excess fluid
and adverse inflammatory factors, either LCOS or pulmonary infection would result in a worse
prognosis. Some studies31 were carried out to break this vicious circle by means of
“perioperative goal‐directed hemodynamic resuscitation therapy,” which was considered to be
useful in reducing adverse complications including LCOS as well as 30‐day mortality in high‐
risk patients undergoing cardiac surgery.
In normal weight BMI group, 24 patients (52.2 %) use diuretics before surgery, while 24
patients (47.8 %) were not taking diuretics before medicine. In overweight BMI group, 25
patients (41 %) use diuretics before surgery, while 36 patients (59 %) were not taking diuretics
before medicine. In normal weight BMI group, 31 patients (67.4 %) use diuretics after surgery,
while 15 patients (32.6 %) were not taking diuretics. In overweight BMI group, 46 patients
(75.4 %) use diuretics after surgery, while 15 patients (24.6 %) were not taking diuretics after
surgery.
While comparing the study of Guang Ju. et al. (2020), A total of 14,154 AKI patients were
included in the data analysis. After PS matching, 4427 pairs of patients were matched between
the patients who received furosemide and those without diuretics treatment. Furosemide was
associated with reduced in-hospital mortality [hazard ratio (HR) 0.67; 95% CI 0.61–
0.74; P < 0.001] and 90-day mortality [HR 0.69; 95% CI 0.64–0.75; P < 0.001], and it was also
associated with the recovery of renal function [HR 1.44; 95% CI 1.31–1.57; P < 0.001] in overall AKI patients. Nevertheless, results illustrated that furosemide was not associated with
43
reduced in-hospital mortality in patients with AKI stage 0–1 defined by UO criteria, AKI stage
2–3 according to SCr criteria, and in those with acute-on-chronic (A-on-C) renal injury.
Furosemide administration was associated with improved short-term survival and recovery of
renal function in critically ill patients with AKI. Furosemide was especially effective in patients
with AKI UO stage 2–3 degree. However, it was not effective in those with AKI SCr stage 2–
3 and chronic kidney disease. The results need to be verified in randomized controlled trials.
In normal weight BMI group, the mean Pre-Operative Hb was 13.43 2, with maximum value
of 19% and minimum value of 10% Pre-Operative Hb. In overweight BMI group, the mean
Pre-Operative Hb was 13.13 2, with maximum value of 16% and minimum value of 7.5%
Pre-Operative Hb. Post-Operative Hb: In normal weight BMI group, the mean postOperative Hb was 11.05 1, with maximum value of 13.7% and minimum value of 8% postOperative Hb. In overweight BMI group, the mean post-Operative Hb was 10.96 1.4, with
maximum value of 14.7% and minimum value of 8% post-Operative Hb.
While comparing the study of Michael H. et al. (2012), We analysed 381 468 mean arterial
pressure (MAP) measurements from 920 consecutive on-pump cardiac surgery patients.
Overall, 19.5% developed AKI which was associated with an 8.2-fold increase in-hospital
mortality. Haemoglobin concentration was an independent risk factor for AKI {odds ratio [OR]
1.16 per g/dL decrease [95% confidence interval (CI) 1.05–1.31]; P = 0.018} with systemic
arterial oxygen saturation and pressure values not adding further strength to such an
association. MAP alone or vasopressor administration was not independently associated with
AKI but volume of red blood cell transfusion was, with its effect being apparent at a
haemoglobin level of >8 g/dL (>5 mmol/L). In patients with severe anaemia (<25th percentile
of lowest haemoglobin), the independent effect of hypotension (>75th percentile of area under
44
the curve MAP <50 mmHg) on AKI was more pronounced [OR 3.36 (95% CI 1.34–8.41); P =
0.010].
Intraoperative avoidance of the extremes of anaemia, especially during severe hypotension and
avoidance of transfusion in patients with hemoglobin levels >8 g/dL (>5 mmol/L) may help
decrease AKI in patients undergoing cardiac surgery and represent targets for future controlled
interventions.
In normal weight BMI group, the mean pre-operative urea was 32.76 12, with maximum
value of 60 mg/dl and minimum value of 18 mg/dl pre-operative urea. In overweight BMI
group, the mean pre-operative urea was 33.30 16, with maximum value of 75 mg/dl and
minimum value of 10 mg/dl pre-operative urea. In normal weight BMI group, the mean postoperative urea was 47.48 40, with maximum value of 174 mg/dl and minimum value of 10
mg/dl post-operative urea. In overweight BMI group, the mean post-operative urea was 45.08
36, with maximum value of 255 mg/dl and minimum value of 14 mg/dl post-operative urea.
In normal weight BMI group, the mean pre-operative creatinine was 0.87 0.27, with
maximum value of 1.4 mg/dl and minimum value of 0.4 mg/dl pre-operative creatinine. In
overweight BMI group, the mean pre-operative creatinine was 0.93 0.3, with maximum
value of 2.2 mg/dl and minimum value of 0.4 mg/dl pre-operative creatinine.
In normal weight BMI group, the mean post-operative creatinine was 1.21 0.4, with
maximum value of 3.9 mg/dl and minimum value of 0.4 mg/dl post-operative creatinine. In
overweight BMI group, the mean post-operative creatinine was 0.93 0.3, with maximum
value of 2.6 mg/dl and minimum value of 0.4 mg/dl post-operative creatinine.
While comparing the study of Yoichi T. et al. (2015), In all, 371 consecutive ADHF patients
were enrolled in the study. AKI was defined as serum creatinine ≥0.3 mg/dl or a 1.5-fold
increase in serum creatinine levels within 48 h. During ADHF therapy, AKI occurred in 99
patients; 55 patients died during the 12-month follow-up period. Grouping patients according
45
to AKI and a median BUN/Cr at admission of 22.1 (non-AKI+low BUN/Cr, non-AKI+high
BUN/Cr, AKI+low BUN/Cr, and AKI+high BUN/Cr groups) revealed higher mortality in the
AKI+high BUN/Cr group (log-rank test, P<0.001). Cox’s proportional hazard analysis
revealed an association between AKI+high BUN/Cr and mortality, whereas the association
with AKI+low BUN/Cr did not reach statistical significance. When patients were grouped
according to AKI and median BUN or creatinine values at admission, AKI was associated with
mortality, regardless of BUN or creatinine.Conclusions:The combination of AKI and elevated
BUN/Cr, but not BUN or creatinine individually, is linked with an increased risk of mortality
in ADHF patients, suggesting that the BUN/Cr is useful for risk stratification of AKI.
In normal weight BMI group, the mean pre-operative urine output was 227.65 196, with
maximum value of 1000 ml and minimum value of no pre-operative urine output recorded. In
overweight BMI group, the mean pre-operative urine output was 223.85 244, with
maximum value of 1000 ml and minimum value of 0 ml pre-operative urine output. In normal
weight BMI group, the mean post-operative urine output was 2837.39 1132, with maximum
value of 4800 ml and minimum value of 200 ml post-operative urine output recorded. In
overweight BMI group, the mean post-operative urine output was 3091.41 847, with
maximum value of 5020 ml and minimum value of 990 ml post-operative urine output.
While comparing the study of Suvi T. et al. (2020), included hourly recorded UO from the
prospective, multicenter FINNAKI study conducted in 16 Finnish intensive care units.
Confounder-adjusted association of oliguria of different severity and duration primarily with
the development of AKI defined by creatinine criterion (Cr-AKI) or renal replacement
therapy (RRT) was assessed. Secondarily, we determined the association of oliguria with 90day mortality. Of the 1966 patients analyzed for the development of AKI, 454 (23.1%) reached
this endpoint. Within this AKI cohort, 312 (68.7%) developed Cr-AKI, 21 (4.6%) commenced
RRT without Cr-AKI, and 121 (26.7%) commenced RRT with Cr-AKI. Episodes of severe
46
oliguria (<0.1 ml/kg/h) for more than 3 h were independently associated with the development
of Cr-AKI or RRT. The shortest periods of consecutive oliguria independently associated with
an increased risk for 90-day mortality were 6–12 h of oliguria from 0.3 to <0.5 ml/kg/h, over
6 h of oliguria from 0.1 to <0.3 ml/kg/h, and severe oliguria lasting over 3 h. Thus, our findings
underlie the importance of hourly UO measurements.
In normal weight BMI group, the mean bypass time was 115.70 43, with maximum value of
227 minutes and minimum value of 57 minutes of bypass time. In overweight BMI group, the
mean bypass time was 121.18 50, with maximum value of 320 minutes and minimum value
of 35 minutes of bypass time.
In normal weight BMI group, the mean cross-clamp time was 61.54 34, with maximum
value of 180 minutes and minimum value of 0 minutes of cross-clamp time. In overweight
BMI group, the mean cross-clamp time was 60.69 31, with maximum value of 320 minutes
and minimum value of 0 minutes of cross-clamp time.
While comparing the study of Avinash B. et al. (2020), The length of time on CPB has been
implicated as an independent risk factor for development of AKI after CPB (AKI-CPB). The 9
independent studies included in the final meta-analysis had 12,466 patients who underwent
CPB. Out of these, 756 patients (6.06%) developed AKI-CPB. In 7 of the 9 studies, the mean
CPB times were statistically longer in the AKI-CPB cohort compared with the control group
(cohort without AKI). The absolute mean differences in CPB time between the 2 groups were
25.65 minutes with the fixed-effects model and 23.18 minutes with the random-effects model.
Longer CPB times are associated with a higher risk of developing AKI-CPB, which, in turn,
has a significant effect on overall mortality as reported by the individual studies.
In normal weight BMI group, 6 patients (13 %) had acute kidney injury, while 40 patients (87
%) were not suffering from acute kidney injury. In overweight BMI group, 7 patients (11.5 %)
had acute kidney injury, while 54 patients (88.5 %) were not suffering from acute kidney injury.
47
While comparing the study of Conlon P. et al. (1999), A total of 2672 of the 2844 patients
underwent isolated coronary artery bypass grafting (CABG) surgery, the remaining 172
underwent valve surgery with or without bypass grafting. Of the CABG patients 7.9%
developed ARF and 0.7% developed ARF-D. The mortality for patients who developed ARF
was 14% (OR 15, P = 0.0001) compared with 1% among those who did not develop ARF. The
mortality for CABG patients who developed ARF-D was 28% (OR 20, P = 0.0001) compared
with 1.8% among those who did not require dialysis. Variables that were significantly
associated with the development of ARF by multivariate analysis included: increased age,
elevated preoperative serum Cr, duration of CPB, presence of a carotid artery bruit, presence
of diabetes, reduced cardiac ejection fraction and increased body weight. Variables
independently associated with ARF-D included serum Cr, duration of CPB, carotid artery bruit
and presence of diabetes. The utility of these models for predicting the development of ARF
and ARF-D was confirmed by bootstrapping techniques. Because of the small number of
patients who underwent valve surgery, none of these variables were significantly associated
with the development of ARF or ARF-D in this group of patients. CONCLUSION: The
development of ARF or ARF-D is associated with a high mortality following CABG surgery.
We have identified perioperative variables, which may be useful in stratifying risk for the
development of ARF.
A study by Chih Chung S. et al. (2017) a total of 188 patients (70 female, mean age 63.7 ± 15.2
years) were enrolled. Comparing with the survivors (n = 124), the non-survivors (n = 64) had
a significantly higher perioperative BW change [3.6 ± 6.1% versus 0.1 ± 8.3%, p = 0.003] but
not the postoperative and pre-RRT BW changes. By using multivariate Cox proportional
hazards model, the independent indicators of 30-day postoperative mortality included
perioperative BW change (p = 0.026) and packed red blood cells transfusion (p = 0.007),
postoperative intra-aortic balloon pump (p = 0.001) and central venous pressure level (p =
48
0.005), as well as heart rate (p = 0.022), sequential organ failure assessment score (p < 0.001),
logistic organ dysfunction score (p = 0.001), and blood total bilirubin level (p = 0.044) at RRT
initiation. The generalized additive models further demonstrated, in a multivariate manner, that
the mortality risk rose significantly during a perioperative BW change of 2% to 15%.
Perioperative BW change was independently associated with an increased risk for 30-day
postoperative mortality in CS patients with RRT-requiring AKI.
49
6.0: Conclusion
Our study has concluded that the post-operative acute kidney injury in the patients undergoing
the cardiopulmonary surgery is based on many pre and intra operative variables like: patient’s
smoking status, weight, gender, hypertension, diabetes, blood urea and creatinine level,
patient’s NYHA class, ejection fraction, hemoglobin, urine output, diuretics use, and intraoperative bypass and cross clamp timings.
However, due to various limitations in our study we cannot specify the bases of BMI and the
post-operative acute kidney injury. Our research has assessed that various above mentioned
variables are also very crucial while stratifying the risk of post-operative acute kidney injury.
50
References:
Amann, Kerstin; Benz, Kerstin (2013). Structural Renal Changes in Obesity and Diabetes.
Seminars in Nephrology, 33(1), 23–33. doi:10.1016/j.semnephrol.2012.12.003
Avinash B. Kumar; Manish Suneja; Emine O. Bayman; Garry D. Weide; Michele Tarasi
(2012). Association Between Postoperative Acute Kidney Injury and Duration of
Cardiopulmonary
Bypass:
A
Meta-Analysis.
,
26(1),
0–69.
doi:10.1053/j.jvca.2011.07.007
Barnaby C Reeves; Raimondo Ascione; Martin H Chamberlain; Gianni D Angelini (2003).
Effect of body mass index on early outcomes in patients undergoing coronary artery
bypass surgery. , 42(4), 668–676. doi:10.1016/s0735-1097(03)00777-0
Billings, F. T.; Pretorius, M.; Schildcrout, J. S.; Mercaldo, N. D.; Byrne, J. G.; Ikizler, T. A.;
Brown, N. J. (2012). Obesity and Oxidative Stress Predict AKI after Cardiac Surgery.
Journal
of
the
American
Society
of
Nephrology,
23(7),
1221–1228.
doi:10.1681/asn.2011090940
Bolton, K., Kremer, P., Rossthorn, N. et al. The effect of gender and age on the association
between weight status and health-related quality of life in Australian adolescents. BMC
Public Health 14, 898 (2014). https://doi.org/10.1186/1471-2458-14-898
Carnethon MR, De Chavez PJD, Biggs ML, et al. Association of Weight Status With Mortality
in
Adults
With
Incident
Diabetes.
doi:10.1001/jama.2012.9282
51
JAMA.
2012;308(6):581–590.
Chagnac, Avry; Weinstein, Talia; Korzets, Asher; Ramadan, Edward; Hirsch, Judith; Gafter,
Uzi (2000). Glomerular hemodynamics in severe obesity. American Journal of
Physiology-Renal
Physiology,
278(5),
F817–F822.
doi:10.1152/ajprenal.2000.278.5.F817
CHIANG, B. N.; PERLMAN, L. V.; EPSTEIN, F. H. (1969). Overweight and Hypertension:
A Review. Circulation, 39(3), 403–421. doi:10.1161/01.CIR.39.3.403 CMAJ April 10,
2007 176 (8) S1-S13; DOI: https://doi.org/10.1503/cmaj.061409
David C.W. Lau, James D. Douketis, Katherine M. Morrison, Irene M. Hramiak, Arya M.
Sharma, Ehud Ur and ; for members of the Obesity Canada Clinical Practice Guidelines
Expert Panel
Jiang, Wuhua; Teng, Jie; Xu, Jiarui; Shen, Bo; Wang, Yimei; Fang, Yi; Zou, Zhouping; Jin,
Jifu; Zhuang, Yamin; Liu, Lan; Luo, Zhe; Wang, Chunsheng; Ding, Xiaoqiang (2016).
Dynamic Predictive Scores for Cardiac Surgery–Associated Acute Kidney Injury. Journal
of the American Heart Association, 5(8), e003754–. doi:10.1161/JAHA.116.003754
Kandler, Kristian; Jensen, Mathias E.; Nilsson, Jens C.; Møller, Christian H.; Steinbrüchel,
Daniel A. (2014). Acute Kidney Injury Is Independently Associated With Higher
Mortality After Cardiac Surgery. Journal of Cardiothoracic and Vascular Anesthesia,
28(6), 1448–1452. doi:10.1053/j.jvca.2014.04.019
Karkouti, K., Grocott, H.P., Hall, R. et al. Interrelationship of preoperative anemia,
intraoperative anemia, and red blood cell transfusion as potentially modifiable risk factors
for acute kidney injury in cardiac surgery: a historical multicentre cohort study. Can J
Anesth/J Can Anesth 62, 377–384 (2015). https://doi.org/10.1007/s12630-014-0302-y
52
Luis F. Ramos, Ayumi Shintani, T. Alp Ikizler, Jonathan Himmelfarb J Am Soc Nephrol. 2008
Mar; 19(3): 593–599. doi: 10.1681/ASN.2007030355 PMCID: PMC2391046
Manoj Kuduvalli, Antony D. Grayson, Aung Y. Oo, Brian M. Fabri, Abbas Rashid, Risk of
morbidity and in-hospital mortality in obese patients undergoing coronary artery bypass
surgery, European Journal of Cardio-Thoracic Surgery, Volume 22, Issue 5, November
2002, Pages 787–793, https://doi.org/10.1016/S1010-7940(02)00448-7
Mariscalco, Giovanni; Wozniak, Marcin J.; Dawson, Alan G.; Serraino, Giuseppe F.; Porter,
Richard; Nath, Mintu; Klersy, Catherine; Kumar, Tracy; Murphy, Gavin J. (2017). Body
Mass Index and Mortality Among Adults Undergoing Cardiac SurgeryClinical
Perspective. Circulation, 135(9), 850–863. doi:10.1161/circulationaha.116.022840
O'Sullivan, Katie E.; Byrne, John S.; Hudson, Amy; Murphy, Anna Marie; Sadlier, Denise M.;
Hurley, John P. (2015). The effect of obesity on acute kidney injury after cardiac surgery.
The Journal of Thoracic and Cardiovascular Surgery, (), S0022522315015457–.
doi:10.1016/j.jtcvs.2015.08.082
P J Conlon, M Stafford-Smith, W D White, M F Newman, S King, M P Winn, K Landolfo,
Acute renal failure following cardiac surgery., Nephrology Dialysis Transplantation,
Volume 14, Issue 5, May 1999, Pages 1158–1162, https://doi.org/10.1093/ndt/14.5.1158
Ricci, Zaccaria; Cruz, Dinna N.; Ronco, Claudio (2011). Classification and staging of acute
kidney injury: beyond the RIFLE and AKIN criteria. Nature Reviews Nephrology, 7(4),
201–208. doi:10.1038/nrneph.2011.14
Robert H. Habib; Anoar Zacharias; Thomas A. Schwann; Christopher J. Riordan; Samuel J.
Durham; Aamir Shah (2005). Effects of Obesity and Small Body Size on Operative and
Long-Term Outcomes of Coronary Artery Bypass Surgery: A Propensity-Matched
Analysis. , 79(6), 0–1986. doi:10.1016/j.athoracsur.2004.11.029
53
Robert H. Habib; Anoar Zacharias; Thomas A. Schwann; Christopher J. Riordan; Samuel J.
Durham; Aamir Shah (2005). Effects of Obesity and Small Body Size on Operative and
Long-Term Outcomes of Coronary Artery Bypass Surgery: A Propensity-Matched
Analysis. , 79(6), 0–1986. doi:10.1016/j.athoracsur.2004.11.029
Rosner, M. H. (2005). Acute Kidney Injury Associated with Cardiac Surgery. Clinical Journal
of the American Society of Nephrology, 1(1), 19–32. doi:10.2215/CJN.00240605
Shiao, Chih-Chung; Huang, Ya-Ting; Lai, Tai-Shuan; Huang, Tao-Min; Wang, Jian-Jhong;
Huang, Chun-Te; Wu, Pei-Chen; Wu, Che-Hsiung; Tsai, I-Jung; Tseng, Li-Jung; Wang,
Chih-Hsien; Chu, Tzong-Shinn; Wu, Kwan-Dun; Wu, Vin-Cent; Wu, Ping-Hsun (2017).
Perioperative body weight change is associated with in-hospital mortality in cardiac
surgical patients with postoperative acute kidney injury. PLOS ONE, 12(11), e0187280–.
doi:10.1371/journal.pone.0187280
Takaya, Yoichi; Yoshihara, Fumiki; Yokoyama, Hiroyuki; Kanzaki, Hideaki; Kitakaze,
Masafumi; Goto, Yoichi; Anzai, Toshihisa; Yasuda, Satoshi; Ogawa, Hisao; Kawano,
Yuhei (2015). Risk Stratification of Acute Kidney Injury Using the Blood Urea
Nitrogen/Creatinine Ratio in Patients With Acute Decompensated Heart Failure.
Circulation Journal, 79(7), 1520–1525. doi:10.1253/circj.cj-14-1360
Tian, J.; Venn, A.; Otahal, P.; Gall, S. (2015). The association between quitting smoking and
weight gain: a systemic review and meta-analysis of prospective cohort studies. Obesity
Reviews, 16(10), 883–901. doi:10.1111/obr.12304
Vaara, Suvi T; Parviainen, Ilkka; Pettilä, Ville; Nisula, Sara; Inkinen, Outi; Uusaro, Ari (2015).
Association of oliguria with the development of acute kidney injury in the critically ill.
Kidney International, (), –. doi:10.1038/ki.2015.269
54
Virani SS, Nambi V, Lee VV, et al. Obesity: an independent predictor of in-hospital
postoperative renal insufficiency among patients undergoing cardiac surgery?. Tex Heart
Inst J. 2009;36(6):540-545.
Weir CB, Jan A. BMI Classification Percentile And Cut Off Points. In: StatPearls. StatPearls
Publishing, Treasure Island (FL); 2021. PMID: 31082114.
Zhao, Gj., Xu, C., Ying, Jc. et al. Association between furosemide administration and outcomes
in critically ill patients with acute kidney injury. Crit Care 24, 75 (2020).
https://doi.org/10.1186/s13054-020-2798-6
55
PERFORMA
The effect of BMI on the kidney function of patients undergoing coronary
artery bypass surgery and valve replacement surgery
Name: _______________
MR no#: _______________
Date of operation__________
Age:
Gender: _______________
BMI: _______________
_______________
Height: ______________
Weight: ___________
Surgery type: Isolated CABG / Isolated Valve / CABG + Valve
Groups:
Normal weight (BMI 18.5-24)
Over weight (BMI 25-30)
RISK FACTORS:
Smoking
Y/N
Asthma
Y/N
Hypertension
Y/N
Chronic Kidney disease
Y/N
Diabetes Mellitus
Y/N
Dyslipidemia
Y/N
CLINICAL VARIABLES:
NYHA: __________
Ejection Fraction: __________
Aortic cross clamp time: __________
Total bypass time: __________
Packed RBC units: __________
Dialysis: Y\N
Variables
Pre-Operative Clinical
Post-Operative Clinical
Assessment
Assessment
Y\N
Y\N
Hb%
Urea (mg/dl)
Creatinine (mg/dl)
Urine output (ml)
Diuretics use
Clinical Outcomes:
Acute Kidney injury
Y\N
56