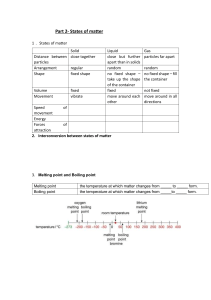
Chemistry Note 14/10/21 Gas Pressure PARTICULES IN A GAS The particles in a gas are spread out far away from each other. They move randomly all the time very freely. If the gas is inside a container, then the particles hit the walls of the container as they move around. Each time a particle collides with the wall, it causes a tiny force. They are huge number of particles colliding with the wall and all this tiny forces add up. We call this gas pressure. Less Space, More Pressure What happens if you squeeze the gas particles into a smaller space? There is the same number of gas particles but they will now hit the walls of the container more often this makes the gas pressure increase. The same thing happens when you squzze more gas particulars into a space.This is what happens when you inflate a football. There are more air particules in the ball, so there are more particules and more frequent collisions with the walls of the ball. This increases the preasure inside the ball