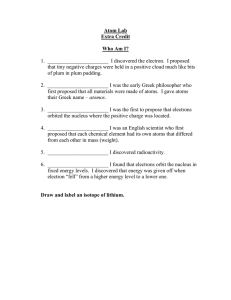
REVIEWER (Active Recall) Atoms – The smallest unit of matter. Molecules - The smallest portion of a substance. Leucippus and Democritus (5th century BCE) - ATOMOS “indivisible” John Dalton - The Solid Sphere/Billiard Ball Model The Modern Atomic Theory of Matter - Matter is made up of small, invisible, and indivisible particles called atoms - Atoms of the same element have the same properties - Atoms cannot be created nor destroyed - Atoms combine in whole number ratios to create compounds Joseph John Thomson - The Plum-Pudding/Raisin Bread Model Discovered the electron using the Cathode Ray Tube experiment Ernest Rutherford - The Nuclear Model Discovered the nucleus using the Gold Foil experiment James Chadwick - Discovered the neutron Niels Bohr - The Planetary Model Erwin Schrodinger - The Quantum Mechanical Model Atomic Number (Z) Atomic Mass (A) Proton (p+) Electron (e-) Neutron (n0)