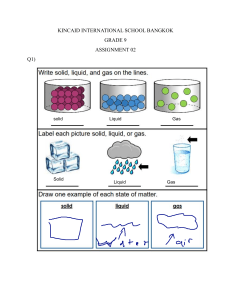
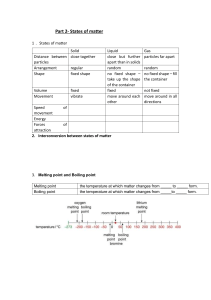
Chemistry 60% Test Isaiah Thomas Secondary School Name: …………………………………………………. Date: …………………………………………………... Form: 4 Section A: Multiple Choice Circle one answer from the questions below. Note: If you change any answer erase the first properly or draw strokes through the previous circle drawn to indicate omission. 1. a. b. c. d. Which one of these has a variable shape and is NOT compressible? Soap Smoke Water Ice 2. a. b. c. d. All of the following statements are true EXCEPT: The boiling point of pure water is 100℃ Only impure substances have a fixed boiling or melting point Whenever a substance is contaminated, its melting point and boiling points are affected Evaporation, unlike boiling, does not occur at a particular temperature 3. a. b. c. d. Kinetic energy is HIGHEST in: Solids Liquids Gases Boiling liquids 4. Which of the following provides direct evidence for the particulate theory of nature of matter? a. Chromatography b. Diffusion c. Distillation d. Melting 5. Which of the following properties is used in fractional distillation? a. Density b. Particle size c. Solubility d. Volatility 1 6. Which of the following can be classified as solutions? I – steel; II – brine; and III - milk a. b. c. d. I only I and II II only II and III 7. Parts of the air can be separated by fractional distillation because: a. Air is a mixture of several gases b. The gases in the air have different boiling points c. Nitrogen is a solvent for all other parts of air d. The parts of air are not in constant composition 8. The sodium ion (Na+ ) has a mass number of 23 and an atomic number of 11. Which two of the following does the ion contain? I12 protons II11 protons III- 12 neutrons IV11 neutrons a. b. c. d. II and III I and IV II and IV I and III 9. The atoms of element X contain 12 electrons. Which of the following elements is in the same group of the Periodic Table as element X? a. Carbon b. Calcium c. Chlorine d. Copper 10. Which of the following metals is a transitional metal? a. Aluminum b. Iron c. Lithium d. Magnesium [10 marks] 2 Section B: Structured Questions Answer ALL of the following questions: 1. Describe the arrangement and movement of particles in each of the three states of matter. Explain what happens to the particles in a liquid during boiling. [6 marks] 2. Food colouring contain a mixture of water-soluble dyes. Devise a method to separate the dyes in a sample of food colouring. Explain how this method works. [6 marks] 3. The table shows information about a substance, X. Melting point Boiling point 356°C -39°C Predict the physical state of substance X at -10°C. [1 mark] 4. The table shows information about a substance, Y. Melting point Boiling point -218°C -183°C Predict the physical state of substance Y at 25°C. [1 mark] 5. Chemists use a model of an atom that consists of three types of sub-atomic particle. These are protons, neutrons and electrons. a. Complete the table below, which shows the properties of these particles. Particle Relative Charge Relative Mass Electrons 1 Protons 0 Neutrons [4 marks] 3 b. Types of atoms and ions vary from each other by the number of sub-atomic particle found in them. What differences are there between 12C and 14 C? [1 mark] c. Define the term 'isotope'. d. [1 mark] Write down the name and the electronic configuration of Cl-. [2 marks] 4