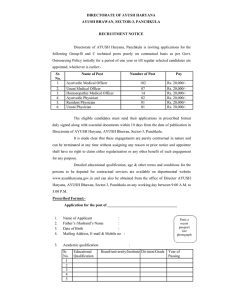
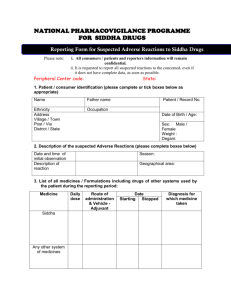
SWAHID JADAV NATH HOMOEOPATHIC MEDICAL COLLEGE & HOSPITAL Under Srimanta Sankardeva University of Health Science,Guwahati Department of Forensic Medicine & Toxicology The Drugs and Cosmetics Act, 1940 is an act of the Parliament of India which regulates---the import, manufacture and distribution of drugs in India. The primary objective of the act is to ensure that the drugs and cosmetics sold in India are safe, effective and conform to state quality standard. The term "drug" as defined in the act, includes various substances, diagnostic, and medical devices. The act defines "cosmetic" as any product that is meant to be applied to the human body for the purpose of beautifying or cleansing. In 1964, the act was amended to include Ayurveda and Unani, Siddha drugs. SECTIONS DEFINE ABOUT… 1 DEFFINATION OF DRUG & COSMETIC 6 THE CENTRAL DRUG LABORATORY 8 STANDARD OF QUALITY 10 Prohibition of import of certain drugs or cosmetics. 16 Standards of quality. 18 Prohibition of manufacture and sale of certain drugs and cosmetics. 21 Inspectors. 33A Chapter not to apply to Ayurvedic, Siddha or Unani drugs. 33C Ayurvedic and Unani Drugs Technical Advisory Board. 33EEB Regulation of manufacture for sale of Ayurvedic, Siddha and Unani drugs. 33G Inspectors.[AYURVEDIC,UNANI,SIDDHA] 34 Offences by companies. Submitted by- MIJANUR RAHMAN University Roll No: Season -2023-24