national pharmacovigilance programme for siddha drugs
advertisement
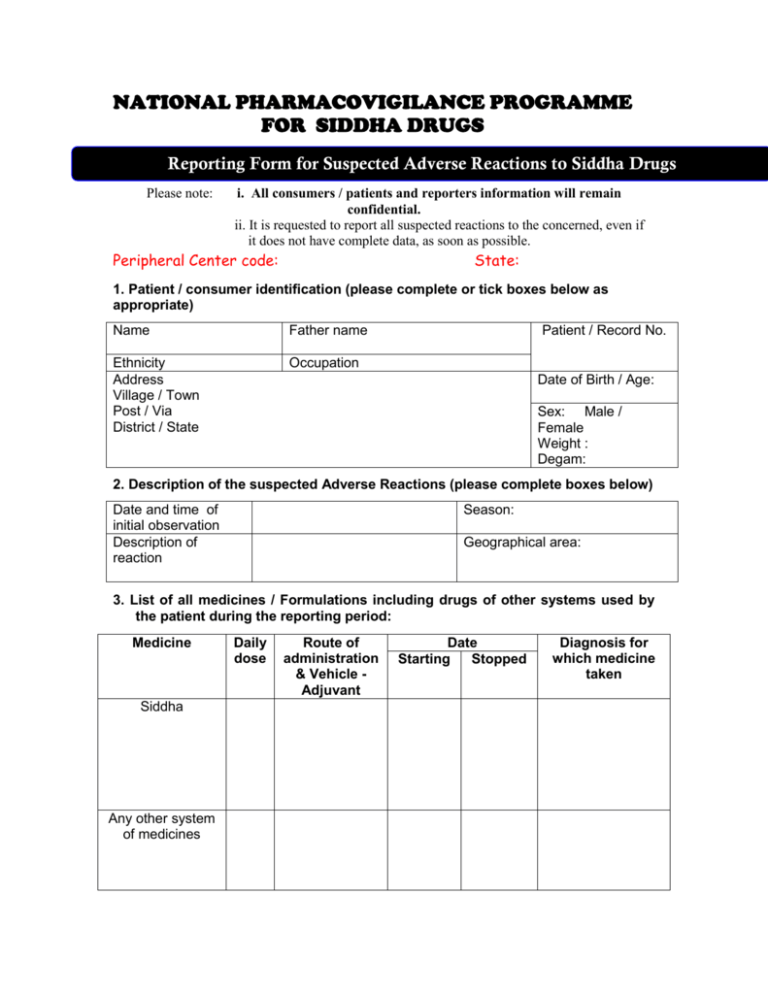
NATIONAL PHARMACOVIGILANCE PROGRAMME FOR SIDDHA DRUGS Reporting Form for Suspected Adverse Reactions to Siddha Drugs Please note: i. All consumers / patients and reporters information will remain confidential. ii. It is requested to report all suspected reactions to the concerned, even if it does not have complete data, as soon as possible. Peripheral Center code: State: 1. Patient / consumer identification (please complete or tick boxes below as appropriate) Name Father name Ethnicity Address Village / Town Post / Via District / State Occupation Patient / Record No. Date of Birth / Age: Sex: Male / Female Weight : Degam: 2. Description of the suspected Adverse Reactions (please complete boxes below) Date and time of initial observation Description of reaction Season: Geographical area: 3. List of all medicines / Formulations including drugs of other systems used by the patient during the reporting period: Medicine Siddha Any other system of medicines Daily dose Route of administration & Vehicle Adjuvant Date Starting Stopped Diagnosis for which medicine taken 4. Brief details of the Siddha Medicine which seems to be toxic : Drug – 1 Details Drug – 2 Drug - 3 a) Name of the medicine b) Manufacturing unit and batch No. and date c) Expiry date d) Purchased and obtained from e) Composition of the formulation / Part of the drug used b) Dietary Restrictions if any c) Whether the drug is consumed under Institutionally qualified medical supervision or used as self medication. d) Any other relevant information. 5. Treatment provided for adverse reaction: 6. The result of the adverse reaction / side effect / untoward effects (please complete the boxes below) Recovered: Not Unknown: Fatal: If Fatal recovered: Date of death: Severe: Yes / No. Reaction abated after drug stopped or dose reduced: Reaction reappeared after re introduction: Was the patient admitted to hospital? If yes, give name and address of hospital 7. Any laboratory investigations done to evaluate other possibilities? If Yes specify: 8. Whether the patient is suffering with any chronic disorders? Hepatic Renal Cardiac Diabetes Malnutrition Any Others 9. H/O previous allergies / Drug reactions: 10. Other illness (please describe): 11. Identification of the reporter: Type (please tick): Nurse / Doctor / Pharmacist / Health worker / Patient / Attendant / Manufacturer / Distributor / Supplier / Any others (please specify) Name: Address: Telephone / E – mail if any : Signature of the reporter: Date: Please send the completed form to: Name & address of the RRCASU / PPC-ASU The Director National Institute of Siddha, (Pharmacovigilance Regional Centre For Siddha Medicine), Tambaram Sanatorium, Chennai-600 047. (O) 044-22381314 Fax : 044 – 22381314 Website : www.nischennai.org Email: nischennaisiddha@yahoo.co.in * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * ** * * * * This filled-in ADR report may be sent within one month of observation /occurrence of ADR Who Can Report? Any Health care professionals like Siddha Doctors / Nurses / Siddha Pharmacists / Patients etc. What to Report? All reactions, Drug interactions, Confidentiality The patient’s identity will be held in strict confidence and protected to the fullest extent. Submission of report will be taken up for remedial measures only not for legal claim











