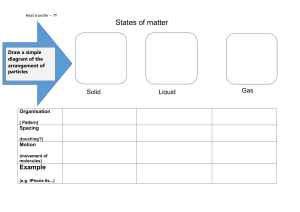
Toribio Minor National High School Quarter 3 Poblacion, Margosatubig, Zamboanga del Sur Science 8 S.Y. 2023-2024 Study Notes Name: _________________________________________________________________ Date: February 15, 2024 MELCS: Explain the properties of solids, liquids, and gases based on the particle nature of matter. (MELC Week 1-2 S8MT-IIIa-b-8) Study Notes: Lesson 1: Particle Nature of Matter Matter has three states namely: solid, liquid and gas. Each state has different arrangement of particles. Solid It has definite shape and volume because its particles are closely packed together in a fixed position. These particles vibrate and are held together by strong attractive forces. It is difficult to compress the particles. Liquid It has a definite volume and takes the shape of the container. Liquid flows easily because its particles have enough space and have less attractive force. The spaces in the particles allows particles to be compressed a little bit. Gas It takes the volume and shape of the container. Gas particles are far from each other that is why they have the weak attractive force and can flow easily. They occupy the entire space available. The large spaces in between particles allows particles to be compressed easily. PROPERTIES OF SOLIDS DENSITY- is the measurement of how tightly a material is packed together. It is defined as the mass per unit volume. Density Symbol: D or ρ Density Formula: ρ =m/V, where ρ is the density, m is the mass of the object and V is the volume of the object. CRYSTALLINE SOLIDS- The individual pieces of crystalline solids are called crystals. Crystalline solid is a solid whose atoms, ions, or molecules are arranged in an orderly, geometric, three dimensional structure. METALLIC SOLIDS- consist of positive metal ions surrounded by a sea of mobile electrons. Mobile electrons make metals good conductor of heat and electricity. AMORPHOUS SOLIDS- is one in which particles are not arranged in a regular, repeating pattern. Amorphous means “ without shape” in Greek. PROPERTIES OF LIQUIDS FLUIDITY-is the ability to flow. Gases and liquids are classified as fluids because they can flow. VISCOSITY- is a measure of the resistance of a liquid to flow. The particles in the liquid are close enough for attractive forces between them. It is the state of being thick, sticky, and semifluid in consistency, due to internal friction - Particle Arrangement: The viscosity of a liquid is influenced by the arrangement of particles. In liquids, particles are in constant motion, and viscosity increases with the degree of intermolecular attraction. - Particle Behavior: Stronger intermolecular forces result in higher viscosity as particles resist sliding past each other more. ***TEMPERATURE- is a measure of the average kinetic energy of the particles in an object. Boiling Point and Melting Point: Particle Arrangement: The strength of intermolecular forces affects these points. In liquids, particles have enough energy to move past each other, but strong intermolecular forces require more energy for a phase transition. Particle Behavior: The boiling point is reached when the vapor pressure equals atmospheric pressure, allowing particles to break free and become a gas. The melting point is when particles gain enough energy to transition from a fixed arrangement in a solid to a more mobile arrangement in a liquid. SURFACE TENSION- is the energy required to increase the surface area of a liquid by a given amount. Particle Arrangement: The surface tension of a liquid is related to the cohesive forces between particles at the liquid's surface. Particle Behavior: At the surface, particles experience a net inward force due to unbalanced cohesive forces, leading to the formation of a "skin" or surface layer. CAPILLARY ACTION- is the ability of a liquid to flow in narrow spaces without the assistance of, or even in opposition to, external forces like gravity. Density - Liquids vary in density. The density of a liquid with a constant volume varies according to the weight. The greater the weight, the higher the density. Particle Arrangement: In liquids, particles are closely packed but not in a fixed, orderly arrangement like in solids. They have more freedom to move around. Particle Behavior: The density of a liquid is determined by the mass of particles in a given volume. The close packing of particles contributes to the relatively high density of liquids compared to gases. Solubility: Particle Arrangement: Solubility depends on the interactions between solute and solvent particles. Particle Behavior: If the intermolecular forces between solute and solvent particles are similar, the substances are more likely to be soluble. Strengthening Unequivocal Response for Excellence 1|5 Toribio Minor National High School Quarter 3 Poblacion, Margosatubig, Zamboanga del Sur Science 8 S.Y. 2023-2024 Study Notes PROPERTIES OF GAS VOLUME- Gases do not have definite volume. This means that a gas will fill whatever volume is available. The volume of gas is measured using a calibrated tube called burette. The SI unit for volume is cubic meter but often use liter (L) and milliliter (mL) or cubic centimeter. Lesson 2: The Phase Change 1. Identify phase changes; 2. Describe how matter undergoes phase change; and 3. Explain physical changes in terms of the arrangement and motion of atoms and molecules. (MELC Week 3-4 S8MTIIIc-d-9) Matter undergoes phase changes. The phase change is a change from one state to another without changing the chemical composition of a substance. There are six phase changes that matter can undergo. Melting is the change of matter from solid state to a liquid state. When liquid state changes back to a solid state, this phase change is called freezing/solidification. Evaporation is changing matter from the liquid state to gas state, while condensation is the change from the gaseous state to liquid state. When solid state directly changes to gas without passing the liquid state, it is called sublimation. In addition, deposition is the change from a gaseous state directly to solid state. Increasing the temperature will result in the increase of kinetic energy (motion) of particles and this will affect the current arrangement of the particles in solid, liquid and gas. As the temperature and the kinetic energy are both increase, the tiny particles move, resulting to a farther distance between the particles. Decreasing the temperature will result in the decrease of kinetic energy (motion) of particles, leading to a closer distance between the particles. The lower the temperature and the kinetic energy, the closer the particles are together. Phase Changes on the Molecular Level: 1. Kinetic Molecular Theory: Describes the behavior of matter at the molecular level. Molecules are in constant motion, and their kinetic energy determines the state of matter. 2. Solid State: Molecular Arrangement: o Molecules are tightly packed in a regular, ordered pattern. o Vibrational motion around fixed positions. Intermolecular Forces: o Strong forces (van der Waals, hydrogen bonding) hold molecules in fixed positions. o Minimal kinetic energy limits movement. 3. Liquid State: Molecular Arrangement: o Molecules are close together but more disordered compared to solids. o Random translational, rotational, and vibrational motion. Intermolecular Forces: o Weaker forces compared to solids, allowing for more movement. o Molecules can slide past each other. Transition to Liquid: o Absorption of heat energy overcomes intermolecular forces. o Molecules gain kinetic energy and transition to a more disordered state. 4. Gaseous State: Molecular Arrangement: o Molecules are widely spaced and move freely. o High kinetic energy with constant motion in all directions. Intermolecular Forces: o Negligible forces between molecules. o Gas particles move independently. Transition to Gas: Absorption of heat energy allows molecules to overcome intermolecular forces. o Increased kinetic energy leads to a transition to the gaseous state. 5. Phase Changes: Melting: o Heat energy disrupts the ordered arrangement of molecules in a solid. o Molecules gain enough energy to break fixed positions and transition to a more disordered state. Vaporization (Boiling/Evaporation): o Heat energy overcomes intermolecular forces in a liquid. o Molecules gain sufficient energy to escape the liquid phase and enter the gaseous state. Condensation: o Heat energy is released as gas molecules lose kinetic energy. o Molecules slow down, re-establish intermolecular forces, and transition back to the liquid state. 6. Temperature and Molecular Kinetics: o Increasing temperature increases molecular kinetic energy. o At specific temperatures (melting point, boiling point), molecules gain enough energy to undergo phase transitions. 7. Sublimation and Deposition: Sublimation: o Solid molecules gain enough energy to transition directly to the gaseous state. Deposition: o Gas molecules lose enough energy to transition directly to the solid state. 8. Equilibrium: At each phase change, there is an equilibrium point where the rate of molecules transitioning from one phase to another is equal in both directions. o Strengthening Unequivocal Response for Excellence 2|5 Toribio Minor National High School Poblacion, Margosatubig, Zamboanga del Sur S.Y. 2023-2024 Quarter 3 Science 8 Worksheets Name: _________________________________________________________________ Date: February 15, 2024 Grade and Section : ______________________________________________________ Score: ___________________ Activity 1. What changes take place? Objective: Identify the phase change in matter. Directions: Answer the questions that follow. Write your answer on the space provided. Lighted candle 1. What is your observation about the candle before it is lighted? ________________________________________________ ________________________________________________________ What is your observation when the candle was lit? ________________________________________________________ How about after putting out the flame of the candle? ________________________________________________________ 2. What state of matter is the lighted candle when it melts? ________________________________________________________ 3. What process takes place when solid state changes to a liquid state?___________________________________________________________________________________ 4. How about when a liquid state changes back to solid? What is the process called? ________________________________________________________________________________________ Letting wet clothes to dry 5. What is your observation on the wet clothes before exposure to the heat of the sun? ___________________________________________________________ *How about during the time of sun exposure? ___________________________________________________________ *Finally, after it was exposed to the sun? ___________________________________________________________ 6. What process takes place when a liquid state changes to a gas state? ___________________________________________________________ 7. How about when the gas state changes back to a liquid? What is the process called? ____________________________________________________________ Toilet deodorizer placed in the comfort room. 8. What is your observation about the size of the toilet deodorizer based from the given conditions? A. Packed toilet deodorizer. ________________________________________ B. Unpacked deodorizer after 5 days. ________________________________ C. Unpacked deodorizer after 15 days.________________________________ 9. What causes the change in its size?__________________________________ _______________________________________________________________ 10. What process takes place when solid state changes to gas state? ______________________________________________________________ Activity 2. Phase Changes Using the Particle Model of Matter Objective: Explain phase changes using the particle model of matter. Directions: Study the illustrations below. Then answer the questions that follow. Write your answer on the space provided. A.1 Refer to Figure 4. 1. What happens to the arrangement of particles of matter in solid, liquid and gas as the temperature increases? ________________________________________ ________________________________________ ________________________________________ ________________________________________ 2. What happens to the kinetic energy of particles of matter in solid, liquid and gas as temperature increases? ________________________________________ ________________________________________ __________________________________________ __________________________________________ Strengthening Unequivocal Response for Excellence 3|5 Toribio Minor National High School Poblacion, Margosatubig, Zamboanga del Sur S.Y. 2023-2024 Quarter 3 Science 8 Worksheets A.2 Refer to Figure 5. 3. What happens to the arrangement of particles of matter in solid, liquid and gas as the temperature decreases? _________________________________________ _________________________________________ _________________________________________ 4. What happens to the kinetic energy of the particles of matter in solid, liquid, and gas as temperature decreases? _________________________________________ _________________________________________ _________________________________________ B. Describe the motion and arrangement of particles in each state as temperature changes. Fill in the table with the correct answer. Write your answers on the space provided. Number 1 is done for you as an example. Phase Change Temperature (increasing or decreasing) Motion (Kinetic Energy) (fast or slow) Arrangement of particles (very close, close or far) increasing fast close 1. Melting (Solid – Liquid) 2. Evaporation (Liquid-Gas) 3. Sublimation (Solid-Gas) 4. Deposition (Gas-Solid) 5. Condensation (Gas-Liquid) 6. Freezing (Liquid-Solid) Activity 3. Fill me in! Directions: Identify the phase change involved by filling in the blanks. Write your answers on the space provided for. Figure 7. The Phase Changes Strengthening Unequivocal Response for Excellence 4|5 Toribio Minor National High School Poblacion, Margosatubig, Zamboanga del Sur S.Y. 2023-2024 Quarter 3 Science 8 Worksheets Activity 4. Phase changes in our environment! Directions: Identify the phase changes shown in the picture. Make a presentation on how water behaves in its states within the water cycle(You can make the presentation by drawing the particles behavior in each phase change.). Answer: ________________________________________________________________________________________________ ________________________________________________________________________________________________ ________________________________________________________________________________________________ ________________________________________________________________________________________________ ________________________________________________________________________________________________ Scoring Rubrics 3 – Discussions do not have misconceptions; with complete scientific evidence. 2 – Discussions do not completely show scientific evidence. 1 – Discussions do not show complete scientific evidence; with misconceptions. 0 – There is no discussion shown. Activity 5. Fill in the blanks Directions: Fill in the blanks with the correct answers. Write your answers on the space provided for. Phase change is the change of state of substance undergoing from one form to another. The chemical composition of the substance is retained even when it undergoes phase change. Matter undergoes phase change due to the change in temperature. The higher the (1)___________ and the kinetic energy, the faster the tiny particles move resulting to the farther distance of the particles from each other. The lower the temperature and the (2) ___________, the particles move slowly leading to a closer distance of the particles. As liquid water evaporates, its temperature (3) ___________and its kinetic energy increases. As the water vapor in the form of clouds condenses, its (4) ___________ decreases and its kinetic energy (5) ___________. When ice melts, its temperature increases and its kinetic energy (6) ___________. As liquid water solidifies, both its temperature and kinetic energy (7) ___________. Source: CO_Q3_Science8_Module 2 Strengthening Unequivocal Response for Excellence 5|5