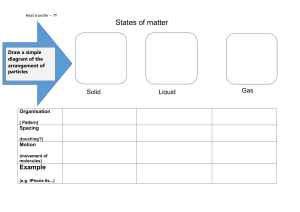
1) The particles are moving freely and at random directions at high speed that is called Brownian motion. 2) 18°C; 192. ii) As the temperature drops from room temperature to the triple point of ethyne, the gas molecules slow down, and their average energy decreases. This happens because temperature and the speed of the particles are connected. When it gets colder, the gas can turn into a liquid or even a solid. iii) 3) P1∗V1 𝑇1 = 𝑃2∗𝑉2 𝑇2 T1= 27°C + 273.15 = 300.15 K T2 = 81°C + 273.15 = 354.15 K 210 𝑘𝑃𝑎∗𝑉1 300.15 𝐾 𝑃2∗𝑉2 = 354.15 𝐾 210 𝑘𝑃𝑎∗354.14𝐾 = 300.15𝐾 P2= 248.37kPa