
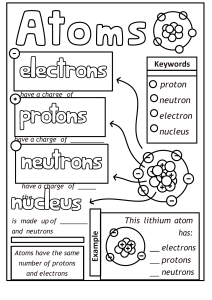
ATOMIC STRUCTURE WHAT IS AN ATOM An atom is defined as the basic building block of an element. It is the smallest particle that shows the chemical features of an element. Atoms are Indivisible particles that join to make Molecules. ATOMS AND ELEMENTS A substance made up of just one type of atom is called an Element. Elements cannot be broken down into anything simpler by chemical reaction. There are 118 known Elements but most of the known mass of the Universe is made up of only two elements (Hydrogen 92% and Helium %) with all the other elements making up the remaining 1 %. The human body contains 65% Oxygen, 18% Carbon, 10 % Hydrogen, 3 %Nitrogen, 2% Calcium and 2% of other elements combined in minute proportions. COMPOUNDS A compound is a substance formed by a chemical combination of two or more elements in fixed proportions. Examples of compounds are HCl, NaCO3, H2SO4. NaCl etc. MOLECULES A Molecule is the smallest particle of a specific Element or Compound that retains the chemical properties of that very element or compound. Two or more atoms held together by chemical bonds. H2O means 1 molecule of water that contains 2 atoms of Hydrogen and 1 atom of Oxygen. Na2CO3 means 1 molecule of Sodium Carbonate containing 2 atoms of Sodium, 1 atom of Carbon and 3 atoms of Oxygen. STRUCTURE OF THE ATOM The atom is made up of three sub atomic particles (Protons, Neutrons and Electrons). It is made up of a Nucleus that is at the center and contains almost all the mass of the whole atom. There are Protons and Neutrons in the Nucleus and Electrons are held in shells around the Nucleus by ELECTROSTATIC FORCE OF ATTRACTION between them and the positively charge Protons. Protons and Neutrons have a Relative mass of 1 while the electron has a negligible mass (1/ 1840). The Proton is positively charge, the Electron negatively charged and the Neutron has no Charge. The simplest atom is the Hydrogen atom which has only one proton in its Nucleus. It is the only atom that has no Neutron. It only contains 1 Proton and 1 Electron. The Lithium atom is the next simplest atom; it has three protons, four Neutrons and three Electrons. The number of Neutrons required to hold the Nucleus together increases as the atomic number increases. An atom of Gold contains 79 protons, 118 Neutrons and 79 Electrons. ATOMIC NUMBER & MASS NUMBER Mass number = Number of protons + number of Neutrons Number of Neutrons = Mass Number -Number of protons Atomic Number = the number of Protons. The atomic number determines the symbol of an Element. It also tells you the number of Electrons in an atom. An atom has no overall charge, because the number of Protons is the same as the number of Electrons. The number of Electrons determines the charge of an Element. The Atomic Number is found on the top left-hand corner of the symbol of the Element while the Mass Number is at the bottom left. Different letters could be used to represent the numbers above. EXERCISE



![[C3-Worksheet Reference] Atoms, Elements & Compounds](http://s3.studylib.net/store/data/025600544_1-ac81b719e1b567117977c0e1deb5ecd6-300x300.png)