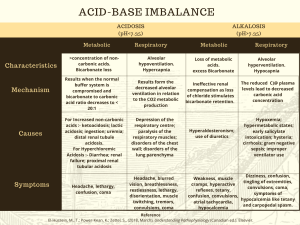
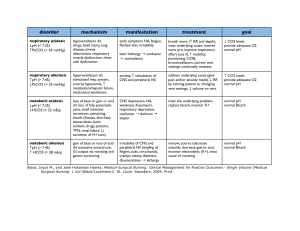
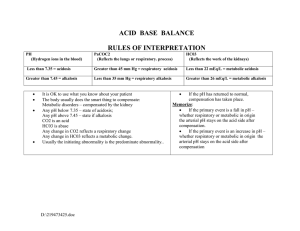
2 questions You answered 2 out of 2 questions correctly on the first attempt. 1. 1. Which two systems in the body are most responsible for regulating the pH in extracellular fluids? You correctly answered: Respiratory and renal systems . Respiratory and renal systems Correct! When the serum pH changes, buffers are used to regulate and maintain an acid–base balance. The respiratory system controls levels of serum carbon dioxide, and the renal system controls levels of serum bicarbonate. Cardiovascular and renal systems Neuromuscular and respiratory systems Cardiovascular and neuromuscular systems 2. 2. Primary acid–base disorders are classified as metabolic or respiratory depending on which factor? You correctly answered: The primary cause of the change in serum pH . The level of absorbed oxygen in the blood The primary cause of the change in serum pH Correct! Respiratory disorders result from changes in the serum carbon dioxide level, and metabolic disorders result from changes in the serum bicarbonate level. The two primary respiratory disorders, respiratory acidosis and respiratory alkalosis, have an increased or decreased carbon dioxide serum level and a normal bicarbonate serum level. The two primary metabolic disorders, metabolic acidosis and metabolic alkalosis, have a normal carbon dioxide serum level and an increased or decreased bicarbonate serum level. The concentration of buffers in the body The measured quantity of intracellular fluids Table 1–1 Common Causes of Primary Acid– Base Imbalances Imbalance Common Causes ↑ Acid production Metabolic acidosis pH < 7.35 HCO3 < 24 mEq/L Critical values pH < 7.20 HCO3 < 10 mEq/L Lactic acidosis Ketoacidosis related to diabetes, starvation, or alcoholism Salicylate toxicity ↓ Acid excretion Renal failure ↑ Bicarbonate loss Diarrhea, ileostomy drainage, intestinal fistula Imbalance Common Causes Biliary or pancreatic fistulas ↑ Chloride Metabolic alkalosis pH > 7.45 HCO3 > 28 mEq/L Critical values pH > 7.60 HCO3 > 40 mEq/L Sodium chloride intravenous (IV) solutions Renal tubular acidosis Carbonic anhydrase inhibitors ↑ Acid loss or excretion Vomiting, gastric suction Hypokalemia ↑ Bicarbonate Alkali ingestion (bicarbonate of soda) Excess bicarbonate administration Acute respiratory acidosis Respiratory acidosis pH < 7.35 PaCO2 > 45 mmHg Critical values pH < 7.20 PaCO2 > 77 mmHg Acute respiratory conditions (pulmonary edema, pneumonia, acute asthma) Opiate overdose Foreign body aspiration Chest trauma Chronic respiratory acidosis Chronic respiratory conditions (COPD, cystic fibrosis) Multiple sclerosis, other neuromuscular diseases Stroke Anxiety-induced hyperventilation Fever Early salicylate intoxication Hyperventilation with mechanical ventilator Respiratory alkalosis pH > 7.45 PaCO2 < 35 mmHg Critical values pH > 7.60 PaCO2 < 20 mmHg Table 1–2 Compensation for Simple Acid– Base Imbalances Primary Disorder Cause Compensation Effect on ABGs ↓ pH Metabolic acidosis Excess nonvolatile acids; bicarbonate deficiency Rate and depth of respirations increase, eliminating additional CO2. ↓ HCO3 ↓ PaCO2 ↑ pH Metabolic alkalosis Bicarbonate excess Rate and depth of respirations decrease; CO2 is ↑ HCO3 retained. ↑ PaCO2 ↓ pH Respiratory acidosis Retained CO2 and excess carbonic acid Kidneys conserve bicarbonate to restore carbonic acid: bicarbonate ratio of 1:20. ↑ PaCO2 ↑ HCO3 ↑ pH Respiratory alkalosis Loss of CO2 and deficient carbonic acid Kidneys excrete bicarbonate and conserve H+ to restore carbonic acid: bicarbonate ratio. ↓ PaCO2 ↓ HCO3 2 questions You answered 2 out of 2 questions correctly on the first attempt. 1. 1. Which buffer system is most associated with the lungs in regulating acid–base balance? You correctly answered: The carbonic acid bicarbonate buffer system . The phosphate hemoglobin binding buffer system The carbonic acid bicarbonate buffer system Correct! The carbonic acid bicarbonate buffer system becomes active when cellular reactions produce the waste product, carbon dioxide, which enters the bloodstream and combines with water to become carbonic acid. The carbonic acid dissociates to bicarbonate ions and hydrogen ions. The bicarbonate helps to neutralize other acids in the body while traveling to the lungs. In the lungs, it converts back to water and carbon dioxide and is released as a waste product. The protein intracellular synthesis buffer system The intracellular chemical buffer system 2. 2. In metabolic acidosis, the buffer system in the kidneys raises the pH value to correct an acid–base imbalance with which actions? You correctly answered: Increasing bicarbonate retention and increasing acid excretion . Increasing bicarbonate retention and increasing acid excretion Correct! To correct metabolic acidosis, the buffer system in the kidneys is stimulated to increase bicarbonate retention and increase acid excretion. This acts to decrease serum carbonic acid concentration and raise the pH value to correct an acid–base imbalance. If the kidneys are overwhelmed and cannot correct the imbalance, further compensation is needed and the respiratory system will increase breathing to expel carbon dioxide in the lungs. Decreasing bicarbonate retention and decreasing acid excretion Slowing breathing down to increase retention of carbon dioxide Increasing breathing to decrease loss of carbon dioxide Metabolic Acidosis ETIOLOGY Conditions that increase nonvolatile acids in the blood (e.g., renal impairment, diabetes mellitus, starvation) Conditions that decrease bicarbonate (e.g., prolonged diarrhea, excessive use of laxatives) Excessive infusion of chloridecontaining IV fluids (e.g., NaCl) Excessive ingestion of acids (e.g., salicylates) Cardiac arrest CLINICAL MANIFESTATIONS Diminished appetite Nausea and vomiting Abdominal pain Weakness Fatigue Headache General malaise Decreasing LOC Dysrhythmia Bradycardia Warm, flushed skin Skeletal problems Hyperventilation (Kussmaul respirations) Dyspnea CLINICAL THERAPIES Monitor ABG values, intake and output, and LOC. Position patient to facilitate chest expansion. Provide oral care for dry mouth. Administer IV sodium bicarbonate carefully if ordered. Treat underlying problem as ordered. Clinical Manifestations and Therapies Metabolic Alkalosis CLINICAL MANIFESTATIONS ETIOLOGY CLINICAL THERAPIES Excessive acid losses due to vomiting or gastric suction Excessive use of potassium-losing diuretics Excessive adrenal corticoid hormones due to: Cushing syndrome Hyperaldosteronism Excessive bicarbonate intake from antacids Parenteral sodium bicarbonate infusion Confusion Decreasing LOC Hyperreflexia Tetany Dysrhythmias Hypotension Seizures Respiratory failure Monitor intake and output closely. Monitor vital signs, especially respirations and LOC. Administer ordered IV fluids carefully. Administer oxygen as ordered. Treat underlying problem. Clinical Manifestations and Therapies Respiratory Acidosis ETIOLOGY Diseases of the airways, such as asthma, chronic obstructive lung disease Disease of the chest CLINICAL MANIFESTATIONS Acute respiratory acidosis: CLINICAL THERAPIES Headache Warm, flushed skin Elevated pulse Blurred vision Assist with identification/treatment of underlying cause. Observe for altered respiratory excursion, rate, and depth. Auscultate breath sounds. Assess LOC and progressive changes. Place in semi-Fowler position or Fowler position as tolerated. ETIOLOGY Drugs that suppress breathing, such as opioids, or alcohol Obstructive sleep apnea CLINICAL MANIFESTATIONS Irritability or altered mental status Decreasing LOC Cardiac dysrhythmias Cardiac arrest Chronic respiratory acidosis: Weakness Dull headache Sleep disturbances with daytime sleepiness Impaired memory Personality changes CLINICAL THERAPIES Encourage the patient with chronic respiratory acidosis to use pursed-lip breathing. Administer oxygen as indicated by mask, cannula, or mechanical ventilation. Increase or decrease respiratory rate on ventilator. Modify respiratory settings as needed. Administer medications as indicated; for example, naloxone hydrochloride (Narcan). Use continuous positive airway pressure (CPAP). Respiratory Alkalosis ETIOLOGY Hyperventilation due to: Brainstem injury Elevated body temperature or fever Extreme anxiety Hypoxia Increased basal metabolic rate Overventilation with a mechanical ventilator Salicylate overdose CLINICAL MANIFESTATIONS Dizziness Numbness and tingling around mouth, hands, and feet Palpitations Dyspnea Chest tightness Anxiety/panic Tremors Tetany Seizures or loss of consciousness CLINICAL THERAPIES Monitor vital signs, LOC, and ABGs. Encourage patient to breathe more slowly; teach breathing and stress reduction techniques. Administer sedative or antianxiety agent as ordered. Monitor ventilator settings. Administer oxygen as ordered. Maintain fluid status.