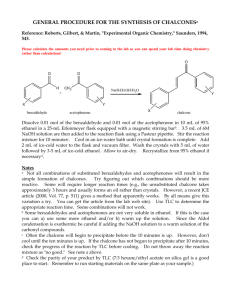
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/350597403 Green synthesis of chalcones derivatives Conference Paper in AIP Conference Proceedings · April 2021 DOI: 10.1063/5.0042002 CITATIONS READS 3 384 5 authors, including: Fera Kurniadewi Hanhan Dianhar Jakarta State University Universitas Negeri Jakarta 41 PUBLICATIONS 267 CITATIONS 22 PUBLICATIONS 52 CITATIONS SEE PROFILE All content following this page was uploaded by Hanhan Dianhar on 21 February 2023. The user has requested enhancement of the downloaded file. SEE PROFILE Green synthesis of chalcones derivatives Cite as: AIP Conference Proceedings 2331, 040020 (2021); https://doi.org/10.1063/5.0042002 Published Online: 02 April 2021 Dewi Septianingtyas, Nahda Zafira, Zulhipri, et al. ARTICLES YOU MAY BE INTERESTED IN Synthesis of new chalcone derivatives and their antimicrobial studies AIP Conference Proceedings 1904, 020013 (2017); https://doi.org/10.1063/1.5011870 Green synthesis of chalcones derivatives as intermediate of flavones and their antibacterial activities AIP Conference Proceedings 1710, 030048 (2016); https://doi.org/10.1063/1.4941514 A simple synthesis of Hantzsch esters derivatives as proton donors in the hydrogenation transfer reaction AIP Conference Proceedings 2331, 040019 (2021); https://doi.org/10.1063/5.0042003 AIP Conference Proceedings 2331, 040020 (2021); https://doi.org/10.1063/5.0042002 © 2021 Author(s). 2331, 040020 Green Synthesis of Chalcones Derivatives Dewi Septianingtyas, Nahda Zafira, Zulhipri, Fera Kurniadewi, and Hanhan Dianhar a) Chemistry Study Program, Faculty of Mathematics and Natural Sciences Universitas Negeri Jakarta, Jalan Rawamangun Muka, Kel. Rawamangun East Jakarta 13220, Indonesia a) Corresponding author: hanhan@unj.ac.id Abstract. Chalcone is a common natural pigment and one of the important intermediates in flavonoid biosynthesis. It has broad bioactivities such as anti-inflammatory, anti-cancer, and anti-fungal properties. In this research, chalcone derivatives were synthesized as a precursor of hydrogenation transfer reaction to afford dihydrochalcones. This study aimed to synthesize chalcone derivatives and determine the effect of hydroxyl substituents qualitatively. Our experiment was carried out to synthesis some chalcone derivatives at room temperature just by stirring process. In this study the synthesis of chalcone derivatives was started from 5 mmol various benzaldehyde and acetophenone in the presence of a 60% KOH catalyst solution then the mixture was stirred for 1.5 hours and allowed to stand for 16 hours. The solution of 10% HCl was added and the formed crystal was then purified by recrystallization. The purity of chalcones yielded was investigated by Thin Layer Chromatography (TLC) and melting point test while the structure was then characterized by UV-vis and NMR spectroscopy. It was found that the optimal stirring time was 1.5 hours, the yield of hydroxyl substituted chalcone was higher than the compound which was not substituted. It can be concluded that the synthesis results in hydroxyl substituted chalcone derivatives have higher yields compared to chalcone derivatives without hydroxyl substitution. INTRODUCTION Chalcone (C15H12O), 1,3-diphenyl-1-propen-1-one is a very important compound in nature. Chalcones contain two aromatic rings (A and B) and one carbon atom α, β-unsaturated. Chalcone, as a precursor of flavonoids and isoflavonoids that are widely found in Indonesian plants. Chemically, chalcone consists of open chain flavonoids in which two aromatic rings join with three carbons in the α, β-unsaturated carbonyl system [1]. Chalcone is synthesized by Claisen-Schmidt condensation, which involves the cross-aldol condensation of aldehydes and ketones with a base or acid catalyst followed by a dehydration reaction. Chalcone is a common natural pigment and one of the important intermediaries in flavonoid biosynthesis [2]. In laboratory synthesis chalcone can be made using the Claisen-Schmidt reaction by reacting acetophenone compounds or their derivatives with benzaldehyde or their derivatives using strong bases such as NaOH, KOH, Ba(OH)2, LiOH.2H2O or NaH as catalysts in polar solvents. Other catalysts that can also be used are sodium phosphate and aluminum-magnesium hydroxide hydrate. Whereas acid catalysts are usually used such as HCl, AlCl3, BF3-Et2O, TiCl4, RuCl3 [3]. Recently, there have been many synthetic studies involving chalcone compounds. From previous research conducted by [4] chalcone synthesis was carried out without the use of solvents, this method produced very high product yields. In the study [4] benzaldehyde and acetophenone were used in the presence of solid NaOH in mortars, with large crude product yields ranging from 81-94%. In 2014 [5] reported that chalcone compound (1,3-diphenyl-2-propen-1-one) and its derivative 4-methoxychalcone could be synthesized by microwave irradiation (400 watt power for 9 minutes) with a catalyst acid (bentonite/montmorillonite) and yield percentage of 1.72% and 5.21%. Several years later [6] reported the synthesis of several chalcone derivatives using two methods, namely the conventional method and the microwave irradiation method. In the research [6] 2-acetyl-5-methyl-furan and aldehyde compounds and alcohol solvents were used. From his research [6] states that most chalcone derivatives have the potential to have cytotoxic and antioxidant activities. The most recent research was in 2019 [7] in which 3,4,4 'trimethoxychalcone was synthesized from 4-methoxy acetophenone and 3,4-dimethoxy benzaldehyde with variations in the amount of NaOH catalyst and time variation. [7] reported that the optimum amount of NaOH catalyst was obtained which was 8 mmol and the optimum reaction time was 3 hours. In this The 2nd Science and Mathematics International Conference (SMIC 2020) AIP Conf. Proc. 2331, 040020-1–040020-4; https://doi.org/10.1063/5.0042002 Published by AIP Publishing. 978-0-7354-4075-3/$30.00 040020-1 research, synthesis of chalcone which can be used as a precursor in the reaction of hydrogenation transfer between chalcone compound and Hantzsch ester will produce dihydrochalcone compound. In this study, benzaldehyde compounds will be reacted with acetophenone compounds in an alkaline atmosphere and chalcone compounds will be produced. In this study, a conventional method was used in which the synthesized compound was purified through the recrystallization process and its purity tested by TLC. METHOD In this study, chalcone derivatives were synthesized from benzaldehyde and acetophenone, where the benzaldehyde compound was varied as benzaldehyde and 4-hydroxy benzaldehyde. This research was conducted at the Research Laboratory of Chemistry Study Program, Faculty of Mathematics and Natural Sciences, State University of Jakarta Synthesis of Chalcone The experiment was started by adding 5 mmol of acetophenone into 5 ml of ethanol in a round bottom flask, then 5 mmol of benzaldehyde was added to the flask. Then to the mixture was added 60% KOH solution, the addition was done by dripping slowly while stirring with a stirrer and then the mixture was stirred with a stirrer for 1.5 hours. When stirring does not involve heating where stirring is carried out at room temperature. After mixing, the mixture is allowed to stand for 14 to 16 hours at room temperature. After being allowed to stand for up to 16 hours the mixture was tested for purity by TLC and melting point test, then the mixture was added with 10% HCl of 1.5 ml. Then the crude product formed is filtered with a Buchner filter and washed with cold aquadest to neutral. Furthermore, recrystallization with cold ethanol is carried out using a Buchner filter until the precipitate is separated from the filtrate. Then the filtered filtrate is evaporated and dried at room temperature. Synthesis 4-Hydroxy Chalcone The experiment was started by adding 5 mmol of acetophenone into 5 ml of ethanol in a round bottom flask, then 5 mmol of 4-hydroxy benzaldehyde was added to the flask. Then to the mixture was added 60% KOH solution, the addition was done by dripping slowly while stirring with a stirrer and then the mixture was stirred with a stirrer for 1.5 hours. When stirring does not involve heating where stirring is carried out at room temperature. After mixing, the mixture is allowed to stand for 14 to 16 hours at room temperature. After being allowed to stand for up to 16 hours the mixture was tested for purity by TLC and melting point test, then the mixture was added with 10% HCl of 1.5 ml. Then the crude product formed is filtered with a Buchner filter and washed with cold aquadest to neutral. Furthermore, recrystallization with cold ethanol is carried out using a Buchner filter until the precipitate is separated from the filtrate. Then the filtered filtrate is evaporated and dried at room temperature. RESULT AND DISCUSSION In this experiment, the synthesis of chalcone derived from benzaldehyde with acetophenone which involved the use of KOH as an alkaline atmosphere was given, while the chalcone synthesis reaction was shown in Figure 1. The benzaldehyde compounds used were varied by 2 types, benzaldehyde, and 4-hydroxy benzaldehyde. This experiment also involved the use of HCl with a concentration of 10%. Before starting the experiment, the first KOH solution is made with a concentration of 60%. The making of the KOH solution is by dissolving 3 grams of KOH solid into 5 ml aquadest. KOH solution is used because KOH is a basic catalyst used in synthesis other than NaOH and KOH tends to work effectively in mild reactions. In KOH solution there is OH- as base nucleophiles (enolates) that have higher nucleophility than acid nucleophiles [8]. CH3 O OHC O KOH + Benzaldehyde H2O Chalcone Acetophenone FIGURE 1. Chalcone Synthesis Reaction from Benzaldehyde and Acetophenone 040020-2 The first experiment was started by putting 5 mmol of acetophenone into 5 ml of ethanol in a round bottom flask, then adding 5 mmol of benzaldehyde to the flask. Then to the mixture was added 60% KOH solution, the addition was done by dripping slowly while stirring with a stirrer and then the mixture was stirred with a stirrer for 1.5 hours. When stirring does not involve heating where stirring is carried out at room temperature [9]. In this experiment room temperature was choose because usually, the synthesis was in high-temperature water (200O to 350OC), so we try the other way with a mild condition (room temperature). After mixing, the mixture becomes bright yellow which is then allowed to stand for 14 to 16 hours at room temperature. Next, the mixture was tested for purity by TLC and melting point. In the TLC test, we used N-Hexane and ethyl acetate (19:1) as an eluent. When plate shined by UV there is one dot, which means the sample was pure and there's no contaminant, from melting point test show that the melting point from the sample was similar to chalcone melting point. After settling for up to 16 hours the solution mixture was added with 10% HCl as much as 1.5 ml, after adding HCl it was seen that in the solution began to form precipitates because the addition of HCl would separate the potassium chalcone salt from the solution [9]. Then the crude product formed is filtered with a Buchner filter and washed with cold aquadest to neutral. Furthermore, recrystallization with cold ethanol is carried out using a Buchner filter until the precipitate is separated from the filtrate. Recrystallization was to purify the product (chalcone) by dissolving in ethanol, so the desired compound or impurities can be removed from the solution. Then the filtered filtrate is evaporated and dried at room temperature, evaporation is carried out to separate the remaining deposits in the first filtered filtrate. Evaporation requires heat (air movement above the sample) that can drive off the solvent from the sample. After that, the sludge was weighed and the resulting weight was 0.1268 grams and the yield was calculated to be 12.19%. The second experiment used a variation of benzaldehyde, 4-hydroxy benzaldehyde reacted with acetophenone. The treatment in this experiment is the same as the previous experiment. In this experiment OH group when positioned within a structure where it can participate in the delocalization of pi electrons, will function as a strong electron donor. Hydroxy substituent as an activating substituent increases the rate of electrophilic substitution and hydroxy can donating an electron to activate the benzene ring toward electrophilic attack, so the yield would be increased. From this second experiment, we obtained a sediment weight of 1.1228 grams and a yield of 99.8%. From the results of these two experiments that were shown in Table 1, it could be seen that the second experiment which involved 4-hydroxy benzaldehyde and acetophenone produced a greater yield. In this case, the addition of hydroxy substituents will increase the yield of the synthesized chalcone compound [10]. TABLE 1. The result of Chalcone Derivatives Synthesis O Acetophenone Variation Benzaldehyde Variation O Crude product: Bright Yellow Weight: 0,1268 gr Yield: 12.19% H O H Crude product: Bright Yellow Weight: 1,1228 gr Yield: 99,8% HO CONCLUSION In this experiment the results of the synthesis were tested by TLC and melting point test, it was found that from both tests the synthesized compound was formed. From the yield obtained, the results of the synthesis 040020-3 involving 4-hydroxy benzaldehyde have a higher yield than the results involving only benzaldehyde in the absence of a hydroxy substituent. From these results, it can be concluded that the addition of hydroxy substituents to benzaldehyde compounds can increase the yield of chalcone compounds formed. ACKNOWLEDGMENT This research was funded by the Dana BLU POK MIPA UNJ 2020 on The Rector’s Letter No. 476/UN39/KU.00.01/2020. REFERENCES 1. 2. 3. S. Syam, S. I. Abdelwahab, M. A. Al-Mamary, S. Mohan, Molecules 17(6), pp. 6179–6195 (2012). A. L. Choudhary, V. Juyal, Int J Pharm Pharm Sci. 3(3), pp. 125–128 (2011). D. K. Mahapatra, S. K. Bharti, V. Asati, Eur J Med Chem 98, pp. 69–114 (2015). [Online]. Available from: http://dx.doi.org/10.1016/j.ejmech.2015.05.004 4. G. Chemistry, J Chem Educ. 81(9), pp. 1345–1347 (2004). 5. Suzana, K. Amalia, M. I. S, M. Rudyanto, H. Poerwono, T. Budiati, J Farm dan Ilmu Kefarmasian Indones. 1(1), pp. 24–27 (2014). 6. M. Rayees Ahmad, V. Girija Sastry, N. Bano, S. Anwar, Arab J Chem 9, pp. S931–S935. [Online]. Available from: http://dx.doi.org/10.1016/j.arabjc.2011.09.002 7. R. Rehana, M. S. Fahreza, M. W. S, ALCHEMY J Penelit Kim. 15(2), p. 228 (2019). 8. D. Dan, V. Dalam, Optimasi Waktu Pengadukan Sintesis Senyawa Kalkon Basa Optimation Stirring Time of Chalcone Synthesis From. :pp. 83–89. 9. SM, .HPS, .SDG, .NSHNM. Trends Appl Sci Res. 2(1), 52–56 (2007). 10. S. S. Shafi and T. Nadu, Synthesis, Characterisation And Antimicrobial Activity Of Some New Chalcones M. Elavarasan, M. Thamizh Thendral and S. Syed Shafi * Department of Chemistry, Thiruvalluvar University, Serkkadu, Vellore - 632115, Tamil Nadu, India. 9(5), pp. 1969–1973 (2018). 040020-4 View publication stats