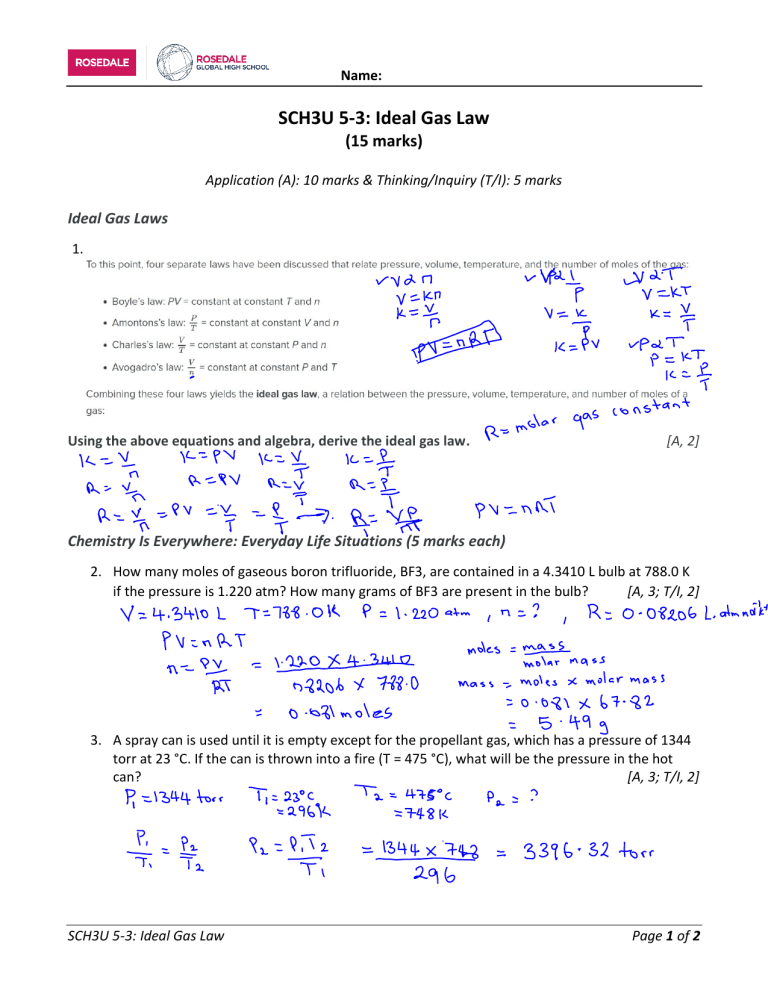
Name: SCH3U 5-3: Ideal Gas Law (15 marks) Application (A): 10 marks & Thinking/Inquiry (T/I): 5 marks Ideal Gas Laws 1. Using the above equations and algebra, derive the ideal gas law. [A, 2] Chemistry Is Everywhere: Everyday Life Situations (5 marks each) 2. How many moles of gaseous boron trifluoride, BF3, are contained in a 4.3410 L bulb at 788.0 K if the pressure is 1.220 atm? How many grams of BF3 are present in the bulb? [A, 3; T/I, 2] 3. A spray can is used until it is empty except for the propellant gas, which has a pressure of 1344 torr at 23 °C. If the can is thrown into a fire (T = 475 °C), what will be the pressure in the hot can? [A, 3; T/I, 2] SCH3U 5-3: Ideal Gas Law Page 1 of 2 Name: Chemistry Is Everywhere: Breathing 4. Breathing (more properly called respiration) is the process by which we draw air into our lungs so that our bodies can take up oxygen from the air. Let us apply the gas laws to breathing. Start by considering pressure. We draw air into our lungs because the diaphragm, a muscle underneath the lungs, moves down to reduce pressure in the lungs, causing external air to rush in to fill the lower-pressure volume. We expel air by the diaphragm pushing against the lungs, increasing pressure inside the lungs and forcing the highpressure air out. Under normal conditions, a pressure difference of only 1 or 2 torr (1 torr = 0.00131579 atm) makes us breathe in and out. Breathing involves pressure differences between the inside of the lungs and the air outside. The pressure differences are only a few torr. How many moles of air do you breathe? Measure your tidal volume (normal breath): [T/I, 1] Breathe in and exhale normally into a balloon. Pinch off the balloon and measure the diameter with at ruler. Then use the following graph to determine your tidal volume. Remember to convert cubic centimeters to meters: 1 cm3 = 0.001L. Consider the room temperature (in Kelvins) and the air pressure can be assumed to be 1 atm. Determine the number of moles that you breathed in. [A, 2] SCH3U 5-3: Ideal Gas Law Page 2 of 2