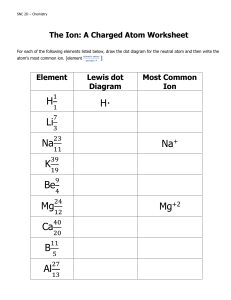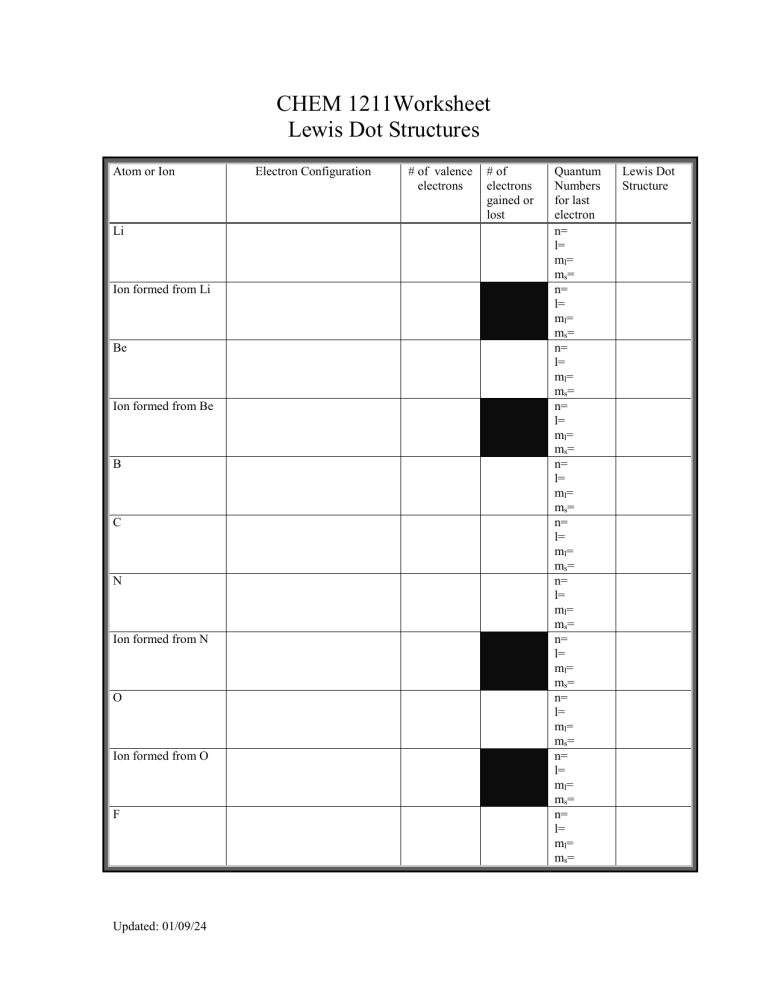
CHEM 1211Worksheet Lewis Dot Structures Atom or Ion Li Ion formed from Li Be Ion formed from Be B C N Ion formed from N O Ion formed from O F Updated: 01/09/24 Electron Configuration # of valence electrons # of electrons gained or lost Quantum Numbers for last electron n= l= ml= ms= n= l= ml= ms= n= l= ml= ms= n= l= ml= ms= n= l= ml= ms= n= l= ml= ms= n= l= ml= ms= n= l= ml= ms= n= l= ml= ms= n= l= ml= ms= n= l= ml= ms= Lewis Dot Structure Atom or Ion Electron Configuration # of valence electrons # of electrons gained or lost Ion formed from F Ne Al Ion formed from Al Ga Ion formed from Ga Se Ion formed from Se Draw the Lewis Dot structures for each of the following ionic compounds: Ionic Compound MgCl2 CaO Na2O Al2S3 Updated: 01/09/24 Lewis Dot Structure Quantum Numbers for last electron n= l= ml= ms= n= l= ml= ms= n= l= ml= ms= n= l= ml= ms= n= l= ml= ms= n= l= ml= ms= n= l= ml= ms= n= l= ml= ms= Lewis Dot Structure LiF BaBr2 K2S CsI Al2O3 Fill in the following chart with the ionic compounds formed from each pair of the following ions: Ion : Li O phosphate N hydroxide Mg Al K Ammonium Place the following salts in order of increasing lattice energy. a) MgO, Rb2O, Na2O b) Lithium Chloride, Potassium Bromide, and Lithium sulfide Compare and contrast ionic and covalent bonding. Updated: 01/09/24 carbonate F What is meant by a polar covalent bond. Draw an illustration and clearly label the important parameters. Fix any inaccuracies contained in each of the following Lewis structures. H N H H a) b) H F H H H B H H c) H H C C O d) O e) N O O Updated: 01/09/24