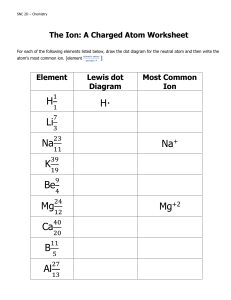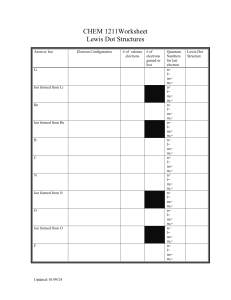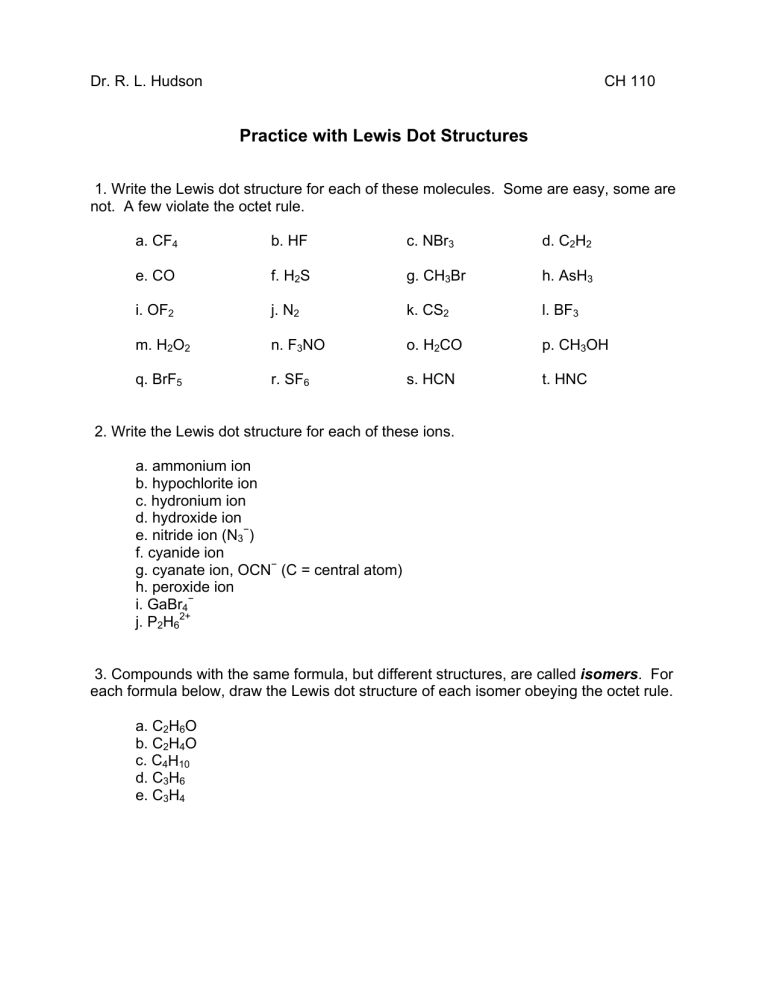
Dr. R. L. Hudson CH 110 Practice with Lewis Dot Structures 1. Write the Lewis dot structure for each of these molecules. Some are easy, some are not. A few violate the octet rule. a. CF4 b. HF c. NBr3 d. C2H2 e. CO f. H2S g. CH3Br h. AsH3 i. OF2 j. N2 k. CS2 l. BF3 m. H2O2 n. F3NO o. H2CO p. CH3OH q. BrF5 r. SF6 s. HCN t. HNC 2. Write the Lewis dot structure for each of these ions. a. ammonium ion b. hypochlorite ion c. hydronium ion d. hydroxide ion e. nitride ion (N3−) f. cyanide ion g. cyanate ion, OCN− (C = central atom) h. peroxide ion i. GaBr4− j. P2H62+ 3. Compounds with the same formula, but different structures, are called isomers. For each formula below, draw the Lewis dot structure of each isomer obeying the octet rule. a. C2H6O b. C2H4O c. C4H10 d. C3H6 e. C3H4