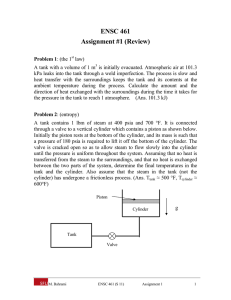
Mass & Energy Analysis of Control Volume ( Open systems unsteady flow) EMM109 Fundamental of Thermal engineering Lecture No. 6_ Dr. Ahmed EL-Seesy Mechanical Engineering Department Benha Faculty of Engineering -Benha University 1 Objectives ❑ Develop the conservation of mass principle. o Apply the conservation of mass principle to various systems including steady- and unsteady flow control volumes. ❑ Apply the first law of thermodynamics as the statement of the conservation of emery principle to control volume. o Identify the energy carried by a fluid stream crossing a control surface as the sum of internal emery, flow work, kinetic energy and potential energy of the fluid and to relate the combination of the internal emery and flow work to the property enthalpy. ❑ Solve energy balance problems for common steady flow devices such as nozzles, compressors, turbines, throttling valves, mixers, and heat exchangers. 2 Conservation of Mass ❑ Mass flow through a cross-sectional area per unit time is called the mass flow rate. Note the dot over the mass symbol indicates a time rate of change. It is expressed as 𝑚. = න 𝜌𝑉. 𝑑𝐴 ❑ If the fluid density and velocity are constant over the flow cross- sectional area, the mass flow rate is Where 𝜌 is the density, kg/𝑚3 , A is the cross sectional are 𝑚2 and V is the Average fluid velocity normal to the area, m/s. 3 Conservation of Mass ❑ The conservation of mass principle for a control volume can be expressed as: 𝑚. 𝑖𝑛 − 𝑚. 𝑜𝑢𝑡 = 𝑑𝑚 𝑠𝑦𝑠𝑡𝑒𝑚 𝑑𝑡 ❑ For a steady state, steady flow process the conservation of mass principle becomes 𝑚. 𝑖𝑛 = 𝑚. 𝑜𝑢𝑡 𝑘𝑔ൗ 𝑠 4 Energy Analysis Of Unsteady-flow Processes ❑ Energy Analysis Of Unsteady-flow Processes: The mass balance for any system undergoing any process can be expressed as: where i inlet, e exit, 1 initial state, and 2 final state of the control volume. Often one or more terms in the equation above are zero. Charging of a rigid tank from a supply line is an unsteadyflow process since it involves changes within the control volume. 5 Energy Analysis Of Unsteadyflow Processes The general energy balance was given earlier as Then the energy balance for a uniform-flow system can be expressed explicitly as The energy equation of a uniformflow system reduces to that of a closed system when all the inlets and exits are closed. 6 EXAMPLE, Charging of a Rigid Tank by Steam A rigid, insulated tank that is initially evacuated is connected through a valve to a supply line that carries steam at 1 MPa and 300°C. Now the valve is opened, and steam is allowed to flow slowly into the tank until the pressure reaches 1 MPa, at which point the valve is closed. Determine the final temperature of the steam in the tank. Solution A valve connecting an initially evacuated tank to a steam line is opened, and steam flows in until the pressure inside rises to the line level. The final temperature in the tank is to be determined. 7 8 EXAMPLE, Cooking with a Pressure Cooker A pressure cooker is a pot that cooks food much faster than ordinary pots by maintaining a higher pressure and temperature during cooking. The pressure inside the pot is controlled by a pressure regulator (the petcock) that keeps the pressure at a constant level by periodically allowing some steam to escape, thus preventing any excess pressure buildup. Pressure cookers, in general, maintain a gage pressure of 2 atm (or 3 atm absolute) inside. Therefore, pressure cookers cook at a temperature of about 133°C (or 271°F) instead of 100°C (or 212°F), cutting the cooking time by as much as 70 percent while minimizing the loss of nutrients. The newer pressure cookers use a spring valve with several pressure settings rather than a weight on the cover A certain pressure cooker has a volume of 6 L and an operating pressure of 75 kPa gage. Initially, it contains 1 kg of water. Heat is supplied to the pressure cooker at a rate of 500 W for 30 min after the operating pressure is reached. Assuming an atmospheric pressure of 100 kPa, determine (a) the temperature at which cooking takes place and (b) the amount of water left in the pressure cooker at the end of the process. 9 Solution: Heat is transferred to a pressure cooker at a specified rate for a specified time period. The cooking temperature and the water remaining in the cooker are to be determined. 10 11 12 13 An insulated, vertical piston–cylinder device initially contains 10 kg of water, 6 kg of which is in the vapor phase. The mass of the piston is such that it maintains a constant pressure of 200 kPa inside the cylinder. Now steam at 0.5 MPa and 350°C is allowed to enter the cylinder from a supply line until all the liquid in the cylinder has vaporized. Determine (a) the final temperature in the cylinder and (b) the mass of the steam that has entered. Answers: (a) 120.2°C, (b) 19.07 kg 14 15 A 0.3-m3 rigid tank is filled with saturated liquid water at 200°C. A valve at the bottom of the tank is opened, and liquid is withdrawn from the tank. Heat is transferred to the water such that the temperature in the tank remains constant. Determine the amount of heat that must be transferred by the time one-half of the total mass has been withdrawn. 16 17 A 2-m3 rigid insulated tank initially containing saturated water vapor at 1 MPa is connected through a valve to a supply line that carries steam at 400°C. Now the valve is opened, and steam is allowed to flow slowly into the tank until the pressure in the tank rises to 2 MPa. At this instant, the tank temperature is measured to be 300°C. Determine the mass of the steam that has entered and the pressure of the steam in the supply line. 18 19