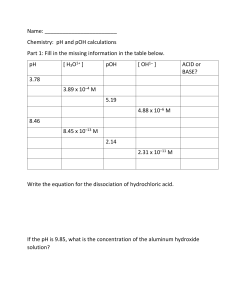
Class Set: Do Not Write on This Sheet Acid and Base Practice Packet --Use your bookl to answer the following questions. Part 1: 1. List all your strong acids and strong bases. 2. Explain how to determine if something is a strong electrolyte. 3. List the following in order of strongest to weakest electrolyte. Justify your choices for each compound. HCl, H2SO4, CH3COOH, CH3OH, NaCl 4. What does pH measure? 5. Define what it means when we say something is a strong acid or base and what it means when something is a weak acid or base. Part 2: 1. Given the following equation: HCl + H2O <==> H3O+ + Cl¯, According to the Bronsted-Lowry theory, a) Identify the acid and conjugate base pair b) Identify the base and conjugate acid pair c) Define a BL base d) Define a BL acid 2. Given the following equation: acts as NH3 + H2O <==> NH4+ + OH¯, According to the Bronsted-Lowry theory, H2O A a base, by receiving a proton B a base, by donating a proton C an acid, by receiving a proton D an acid, by donating a proton 3. Define an Arrhenius acid and give examples. Explain how an Arrhenius acid is different from a Lewis acid. 5. Fill in the table below. Equation Acid Base Conjugate Conjugate Base Acid HF + H2O F- + H3O+ HSO4- + NH3 SO42- + NH4+ C2H3O2- + HCl HC2H3O2 + Cl- HNO2 + H2O H3O+ + NO2- HCN + H2O H3O+ + CN- Chemistry: pH and pOH calculations Part 3: Fill in the missing information in the table below. pH [ H3O1+ ] pOH [ OH1– ] 3.78 3.89 x 10–4 M 5.19 4.88 x 10–6 M 8.46 8.45 x 10–13 M 2.14 2.31 x 10–11 M 10.91 7.49 x 10–6 M 9.94 2.57 x 10–8 M 7.05 4.73 x 10–10 M 1.33 9.87 x 10–3 M ACID or BASE? Part 4: For each of the problems below, assume 100% dissociation. 1. Find the pH of a 0.00476 M hydrochloric acid solution. 2. Find the pH of a solution that contains 3.25 g of H2SO4 dissolved in 2.75 liters of solution. 3. Find the pH of a 0.000841 M solution of sodium hydroxide. What is the pOH of a 3.4x10-6 M solution? 4. If the pH is 9.85, what is the concentration of the aluminum hydroxide solution? 5. A 5.34x10-4 M solution of calcium hydroxide has a pH of what? 6. What is the hydronium (H+) ion concentration of a HBr solution of pH 5.63. 7. What is the hydroxide ion concentration of a sodium hydroxide solution with a pH of 8.94? 8. What is the pOH of a solution that has a [H+] of 0.000053 M? 9. . What is the pOH of a solution that has a [H+] of 9.8x10-5 M? 10. List the following in order of highest to lowest hydroxide ion concentration. pH = 2, pH=5, pH=7.3, pH = 9.8