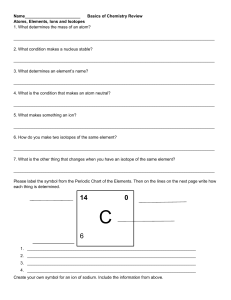
Question Booklet 1 Chemistry C1 1. The table below gives the composition of six particles which are either atoms or ions. (a) Which particles are atoms? Explain your choice. ........................................................................................................................................................ ...............................................................................................................................................[2] (b) Which particle is a negative ion and why has this particle got a negative charge? .................................................................................................................................................... .............................................................................................................................................. [2] (c) Which particles are positive ions? .............................................................................................................................................. [1] (d) Explain why particle A and particle D are isotopes. .................................................................................................................................................... .............................................................................................................................................. [2] [Total: 7] 2. In the Periodic Table, the elements are arranged in columns called Groups and in rows called Periods. (a) Complete the table for some of the elements in Period 3. group number 1 2 13 14 15 16 17 symbol Na Mg Al Si P S Cl number of electrons (b) Lithium has two naturally-occurring isotopes. These can be written as: (i) Describe the difference between these isotopes ........................................................................................................................................ [1] 3. The symbols for two isotopes of iron are shown below. (i) How do these two isotopes differ in their atomic structure? .............................................................................................................................................. [1] (ii) Determine the number of neutrons present in one atom of the isotope Fe-57. .............................................................................................................................................. [1] (iii) Determine the number of electrons in one Fe3+ ion? .............................................................................................................................................. [1] 4. The table gives the composition of three particles. What is the evidence in the table for each of the following? Particle A is an atom. .............................................................................................................................................. [1] A, B and C are all particles of the same element. .............................................................................................................................................. [2] Particles A and C are isotopes of the same element. .............................................................................................................................................. [1] 5. 6. 7. 8. C =12 Abundance 98.9 % C=13 1.06 % C=14 0.04 % Find Relative Atomic Mass of Carbon ( 2dp answer required )