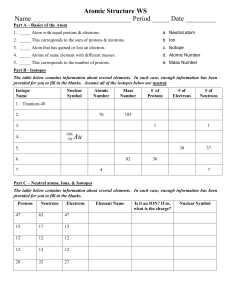
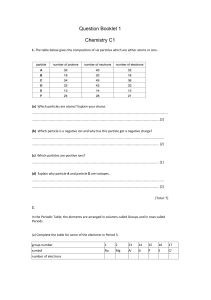
1. The following particles all have the same electronic arrangement of 2, 8, 8: K+, Ca2+, Cl-, Ar, S2-. a. Which particle has the same number of protons as electrons? b. Which particle has 20 protons but only 18 electrons? c. Which particle has one more electron than protons? d. Which particle is an atom? 2. An atom of phosphorus has an atomic number of 15 and a mass number of 31. Give the number of protons, neutrons and electrons in an atom of phosphorus. 3. Silicon’s three most stable isotopes are 28Si, 29Si and 30Si. a. Define the term “isotope” b. Silicon has a relative atomic mass of 28.1. Choose the most abundant isotope from the list of silicon’s three most stable isotopes. Explain your answer. 4. 32S and 34S are the two most common isotopes of Sulphur. Describe the similarities and differences between these two isotopes. 5. Element X has an atomic number of 17. a. Which group of the periodic table will this element belong to? b. Give the atomic number of the element that would be directly above element X in the periodic table. 6. An element reacts by losing its outer shell electrons to form cations. Is this element a metal or a non-metal? 7. What do the noble gas group all have in common? 8. Stearic acid is used for making soap. The melting point and the boiling points of stearic acid are 70°C and 287°C respectively. Sketch a graph to show the changes in temperature when molten stearic acid is cooled to room temperature. 9. Classify the following changes of state into two groups: Heat taken in and heat given out by the particles: sublimation, condensation, evaporation, boiling, melting and freezing. 10. Why does the scent of perfume spread? and why does the scent wear off faster in warm weather than in cold?