Chemistry Lesson Plan: Experimental Techniques & Past Paper Practice
advertisement
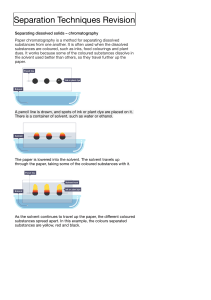
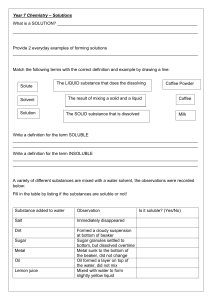
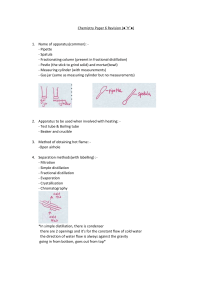
Teacher The Science School - LESSON PLAN Name Javaria Number of Subject Chemistry Class 11 lessons per 8 Date 20 -24 nov 2023 week Topic Past paper practice Experimental Techniques Experimental design CAIE specification 1 Name appropriate apparatus for the measurement of time, temperature, mass and volume, including: (a) stopwatches (b) thermometers (c) balances (d) burettes (e) volumetric pipettes (f) measuring cylinders (g) gas syringes 2 Suggest advantages and disadvantages of experimental methods and apparatus 3 Describe a: Subtopic Instruments and their accuracy and use Chromatography Filtration Simple and fractional distillation Separating funnel (a) solvent as a substance that dissolves a solute (b) solute as a substance that is dissolved in a solvent (c) solution as a mixture of one or more solutes dissolved in a solvent (d) saturated solution as a solution containing the maximum concentration of a solute dissolved in the solvent at a specified temperature (e) residue as a substance that remains after evaporation, distillation, filtration or any similar process (f) filtrate as a liquid or solution that has passed through a filter Chromatography 1 Describe how paper chromatography is used to separate mixtures of soluble coloured substances, using a suitable solvent 2 Interpret simple chromatograms to identify: (a) unknown substances by comparison with known substances (a) pure and impure substances (b) 3 Describe how paper chromatography is used (c) to separate mixtures of soluble colourless (d) substances, using a suitable solvent and a (e) locating agent (f) Knowledge of specific locating agents is not (g) required (h) 4 State and use the equation for Rf: (i) Rf = distance travelled by substance distance travelled by solvent Separation and purification 1 Describe and explain methods of separation and purification using: (a) a suitable solvent (b) filtration (c) crystallisation (d) simple distillation (e) fractional distillation 2 Suggest suitable separation and purification techniques, given information about the substances involved 3 Identify substances and assess their purity using melting point and boiling point information All Chemistry 0620 syllabus objectives will be covered via different conceptual questions while past paper practice October November 2018 paper 2 ,4 and 6 Objectives Actions of the pupils and the Vocabular Assessment Tim Res teacher in the lesson y Tasks (formal / e ourc informal) Block 1 and 2 Block 1 and 2 acid Concepts Theory paper 2018 may June V1 base What is chemical bonding? paper 41 marking key discussion Alkali 0620 October November paper 21 of 2018 Homework es Wor 40+4 0 min kboo k Home task Completion of all theory questions 1- What are different types of and correction which was assigned chemical bonding? as home task in previous week. What are macromolecules? What is reduction? What is oxidation? What are salts? Home task: Completion of all theory questions 1-8 of chapter experimental What are cations? What are anions? What is atom Workbook link given below What are acids and https://docs.google.com/docume techniques page number 102-108 bases nt/d/1rTLKaNlQuHAQtYdYnhg0B8 What are isotopes? gh5kPF_lV9/edit?usp=sharing&o What is reactivity series uid=105629946120097931920&rtp of metals? of=true&sd=true What is method for extraction of metals? Skills Describe ionic bonding Describe ionic lattice Draw dot and cross diagram for ionic and covalently bonded structures. Block 3 and 4 Marking key discussion and Indicator End point Salts Neutralizati on Concentrati on Pipette Burette Precipitate Soluble Insoluble correction of home task Ionic assigned. equation Class task Crystallizati on 8 of chapter tested with cobalt (II) chloride paper. Observation s Drops of aqueous sodium hydroxide were added to the first portion of solution N until a change was seen.Obser vations experimenta l techniques page number 102-108 of all theory questions 18 of chapter experimenta l techniques page number 102-108 Identify solid O. Plan an experiment to obtain pure crystals of potassium sulfate from sulfuric acid and potassium Completion paper 6 (atp) 40mi October n November 2018 61 Complete the incomplete work and Describe the physical and Completion of atp questions 1 2 and Saturated chemical properties of ionic 3 from workbook page number 109- solution and covalent bonded 112 compounds. State different examples of macromolecules Describe arrangement of elements in nt/d/1rTLKaNlQuHAQtYdYnhg0B8 Determine the link between uid=105629946120097931920&rtp paper the physical properties of of=true&sd=true structure. revise n organic chemistry . home task : Lime water Determine the oxidized and paper 6 (atp) October November reduced species in given 2018 61 Determine the oxidizing 40mi Milky chemical reaction Bleach Red litmus macromolecules with their https://docs.google.com/docume Residue gh5kPF_lV9/edit?usp=sharing&o macromolecules Workbook link given below Filtrate hydroxide solution. You are provided with common laboratory apparatus. Moles molar Block 5 and 6 volume and reducing agent in given chemical reaction State the color changes Marking key discussion and concentratio 40mi correction of Atp questions of n n+40 involved when oxidizing Experimental techniques and of and reducing agents are paper 6, October November 2018 used, variant 61 State observation and identify the given salt through chemical tests min empirical molecular formula Describe different methods of metal extraction Block 3,4,5,6 Concepts Block 7 and 8 reactant apparatus used in laboratory? What are mixtures? What are compounds? 45+3 Student will solve the past year paper multiple choice question 2018 (V1) October November paper 21 Followed by marking key discussion What are different limiting and correction through selfassessment percentage yield Addition Substitution Halogenatio n Home task Cracking What are different Complete the incomplete work and Hydrogenati experimental techniques revise organic chemistry on used to separate mixtures Combustion Skills Alcohols State purpose of apparatus used Fermentatio n State the accuracy of different apparatus used Catalytic Name the technique used hydrogenati to separate the given on mixture Describe crystallization Describe filtration Salt formation 5 min Describe simple and fractional distillation Describe chromatography Describe separation of immiscible liquids Block7 and 8 What are acids? What are bases? What are oxides? Skills: State different types of oxides State their solubility in acids and bases Describe separation of mixture of any two oxides given Describe acids with examples Describe bases with examples Differentiate between bases and alkalis State the Difference between weak and strong acids/bases Describe some chemical properties of acids and bases Block 7 and 8 What are salts? What are soluble salts? What are insoluble salts? What are Polymers? What is polymerization? Skills State solubility rules of different salts Describe method of preparation of soluble and insoluble salts Describe method of soluble salts preparation. Describe polymers State difference between condensation and addition polymerization State the examples of both types of polymerizations with drawing structures Identify the monomers of both addition and condensation polymers in given polymer Draw structures of proteins showing the linkages Attitudes Content to be covered Book: Chemistry by Christopher N Prescott Experimental Techniques (7-21) Book: Chemistry Course book by Richard Harwood and Ian Lodge Experimental techniques (1-25)