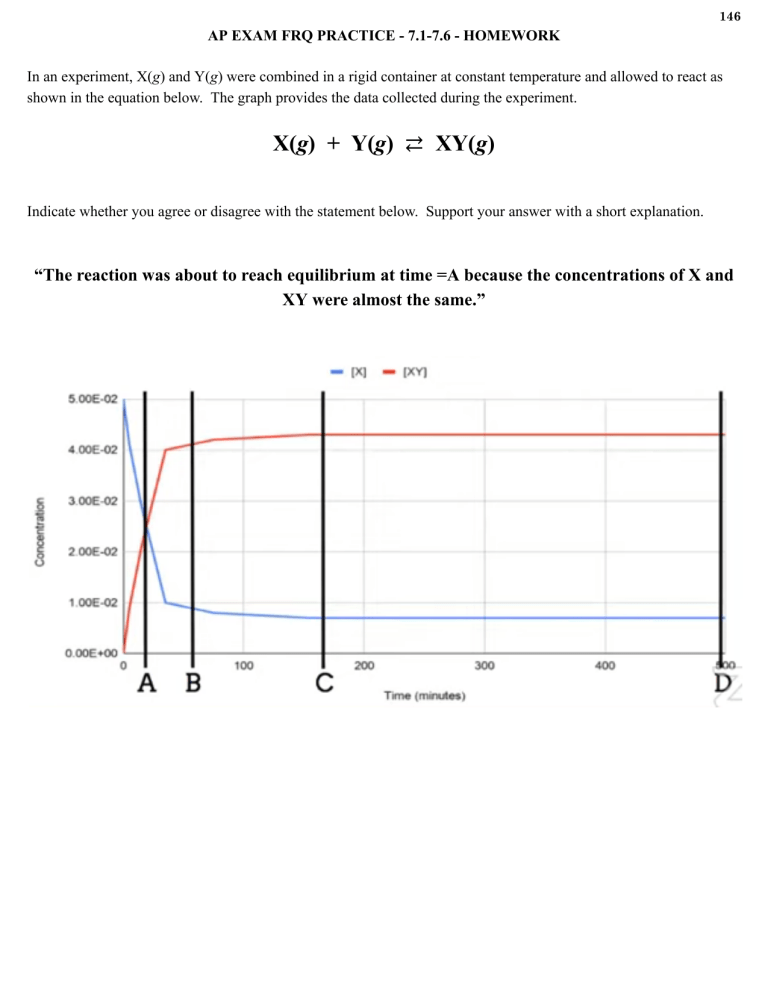
146 AP EXAM FRQ PRACTICE - 7.1-7.6 - HOMEWORK In an experiment, X(g) and Y(g) were combined in a rigid container at constant temperature and allowed to react as shown in the equation below. The graph provides the data collected during the experiment. X(g) + Y(g) ⇄ XY(g) Indicate whether you agree or disagree with the statement below. Support your answer with a short explanation. “The reaction was about to reach equilibrium at time =A because the concentrations of X and XY were almost the same.” 147 AP EXAM FRQ PRACTICE - 7.1-7.6 - HOMEWORK (CONTINUED) An equimolar mixture of X(g) and Y(g) is placed inside a rigid container at constant temperature. Each particle diagram below represents the changes that occur over time. Draw a particle diagram in the box for t = 400 s that is consistent with the conclusion that equilibrium was reached for this reaction after 300 seconds, but before 400 seconds. N2(g) + O2(g) ⇄ 2 NO(g) 3. At high temperatures, N2(g) and O2(g) can react to produce nitrogen monoxide, NO(g), as represented by the equation above. (a) Write the expression for the equilibrium constant, KP, for the forward reaction. (b) A student injects N2(g) and O2(g) into a previously evacuated, rigid vessel and raises the temperature of the vessel to 2000oC. At this temperature the initial partial pressures of N2(g) and O2(g) are 6.01 atm and 1.61 atm, respectively. The system is allowed to reach equilibrium. The partial pressure of NO(g) at equilibrium is 0.122 atm. Calculate the value of KP. 158 AP EXAM FRQ PRACTICE 7.7 - 7.10 - HOMEWORK CaCO3(s) ⇄ CaO(s) + CO2(g) When heated, calcium carbonate decomposes according to the equation above. In a study of the decomposition of calcium carbonate, a student added a 50.0 g sample of powdered CaCO3(s) to a 1.00 L rigid container. The student sealed the container, pumped out all of the gases, then heated the container in an oven at 1100 K. As the container was heated, the total pressure of the CO2(g) in the container was measured over time. The data are graphed and plotted in graph below. The student repeated the experiment, but this time the student uses a 100.0 g sample of powdered CaCO3(s). In this experiment, the final pressure in the container was 1.04 atm, which was the same final pressure as in the first experiment. (a) Calculate the number of moles of CO2(g) present in the container after 20 minutes of heating. (b) The student claimed that the final pressure in the container in each experiment became constant because all of the CaCO3(s) had decomposed. Based on the data in the experiments, do you agree with this claim? Explain. (c) After 20 minutes some CO2(g) was injected into the container, initially raising the pressure to 1.5 atm. Would the final pressure inside the container be less than, greater than, or equal to 1.04 atm. Explain your reasoning. (d) Are there sufficient data obtainer in the experiments to determine the value of the equilibrium constant, KP, for the decomposition of CaCO3(s) at 1100 K? Justify your answer. 159 AP EXAM FRQ PRACTICE 7.7 - 7.10 - HOMEWORK (CONTINUED) Ba2+(aq) + EDTA4- (aq) ⇄ Ba(EDTA)2- (aq) K = 7.7 × 107 The polyatomic ion C10H12N2O84- is commonly abbreviated as EDTA4-. The ion can form complexes with metal ions in aqueous solutions. A complex of EDTA4- with Ba2+ ion forms according to the equation above. A 50.0 mL volume of a solution that has an EDTA4-(aq) concentration of 0.30 M is mixed with 50.0 mL of 0.20 M Ba(NO3)2 to produce 100.0 mL of solution. a. Considering the value of K for the reaction, determine the concentration of Ba(EDTA)2- (aq) in the 100.0 mL of solution. Justify your answer. b. The solution is diluted with distilled water to a total volume of 1.00 L. After equilibrium has been reestablished, is the number of moles Ba2+(aq) of present in the solution greater than, less than, or equal to the number of moles of Ba2+(aq) present in the original solution before it was diluted? Justify your answer. 169 AP EXAM FRQ PRACTICE 7.11 - 7.13 - HOMEWORK 1. Answer the following questions about the solubility of some fluoride salts of alkaline earth metals. (a) A student prepares 100. mL of a saturated solution of MgF2 by adding 0.50 g of solid MgF2 to 100. mL of distilled water at 25oC and stirring until no more solid dissolves. (Assume that the volume of the undissolved MgF2 is negligibly small.) The saturated solution is analyzed, and it is determined that [F-] in the solution is 2.4 × 10-3 M. (i) Write the chemical equation for the dissolving of solid MgF2 in water. (ii) Calculate the number of moles that MgF2 that dissolved. (iii) Determine the value of the solubility-product constant, Ksp, for MgF2 at 25oC. (b) A beaker contains 500. mL of a solution in which both Ca2+(aq) and Ba2+(aq) are present at a concentration of 0.10 M at 25oC. A student intends to separate the ions by adding 0.20 M NaF solution one drop at a time from a buret. At 25cC the value of Ksp for CaF2 is 3.5 × 10-11; the value of Ksp for BaF2 is 1.8 × 10-6. (i) Which salt will precipitate first, CaF2 or BaF2? Justify your answer. For parts (b)(ii) and (b)(iii) below, assume that the addition of the NaF solution does not significantly affect the total volume of the liquid in the beaker. (ii) Calculate the minimum concentration of F-(aq) necessary to initiate precipitation of the salt selected in part (b)(i). (iii) Calculate the minimum volume of 0.20 M NaF that must be added to the beaker to initiate precipitation of the salt selected in part (b)(i). (c) There are several ways to dissolve salts that have limited solubility. Describe one procedure to redissolve the precipitate formed in part (b).