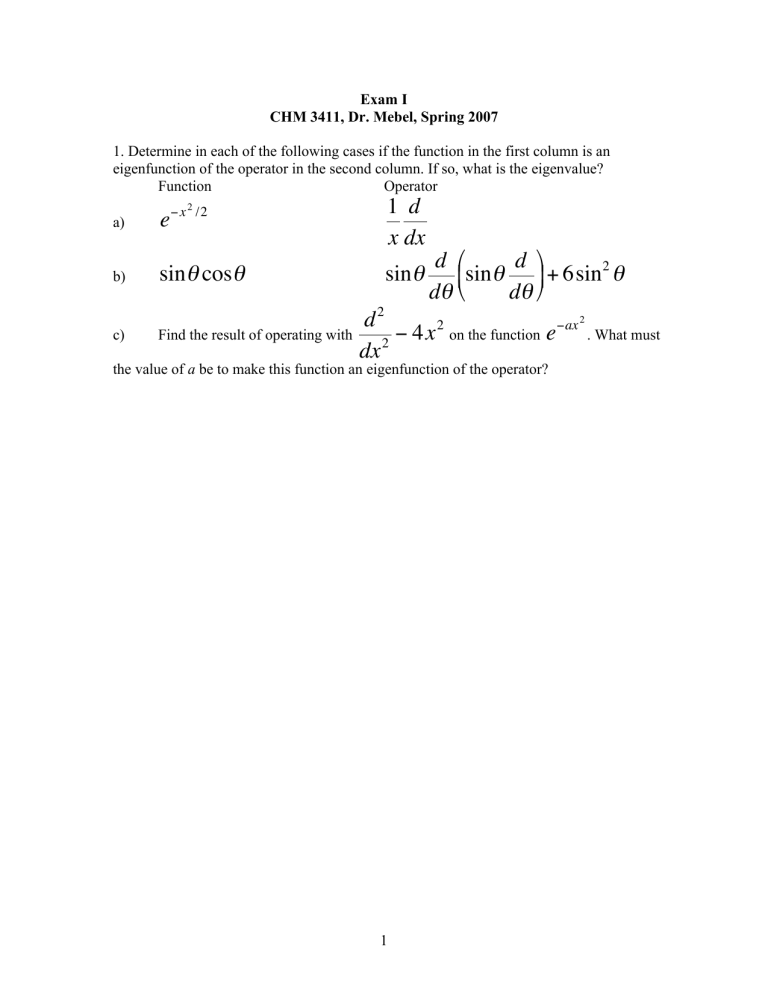
Exam I CHM 3411, Dr. Mebel, Spring 2007 1. Determine in each of the following cases if the function in the first column is an eigenfunction of the operator in the second column. If so, what is the eigenvalue? Function Operator a) e b) sinθ cosθ € c) € 1 d x dx − x 2 /2 sinθ € with Find the result of operating d d 2 sinθ + 6sin θ dθ dθ d2 −ax 2 2 on the function e . What must − 4x 2 dx the value of a be to make this function an eigenfunction of the operator? € € € 1 −r / a 2. (a) Normalize the wavefunction e cosθ sin φ over the interval 0 < r < ∞, 0 < θ < π, 0 < φ < 2π. (b) Calculate the mean radius r (expectation value <r>) for this wavefunction. € 2 3. The force constant for a H19F molecule is 966 N m-1. (a) Calculate the zero point vibrational energy for this molecule considering it as a harmonic oscillator. (b) Calculate the wavenumber (in cm-1) of the light needed to excite this molecule from the ground state to the first excited state. [The mass to use in the expression for the vibrational frequency of a diatomic molecule is the effective mass µ= m A mB , where mA and mB are the masses of individual atoms]. m A + mB Assuming that the force constant of the bond does not change with isotope substitution, repeat your calculations for a D19F molecule, where D is deuterium. € 3 4. A gas phase 1H16O radical rotates in a three-dimensional space. It is known that the energy difference between the two lowest rotational energy levels of this radical is 7.547×10-22 J. Calculate the bond length R for this radical, using the fact that its moment of inertia is I = µR2 , where µ = m H mO m H + mO € € € € 4 and R is the bond length. 5. Predict wavenumbers for the first four transitions in the Paschen series (n1 = 3) for the Be3+ ion. What is the ionization energy of this ion from the ground electronic state? 5