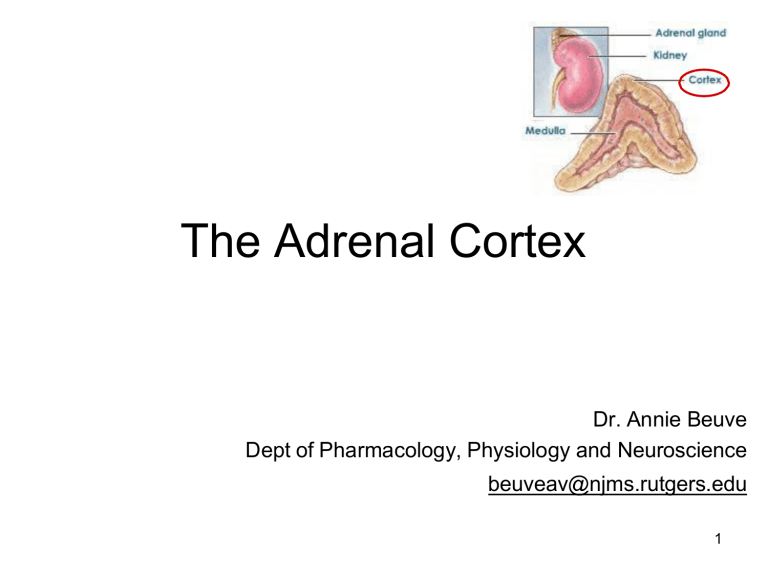
The Adrenal Cortex Dr. Annie Beuve Dept of Pharmacology, Physiology and Neuroscience beuveav@njms.rutgers.edu 1 The major hormones secreted by the adrenal cortex are CORTISOL which is a glucocorticoid ALDOSTERONE which is a mineralocorticoid androgens are also secreted by the adrenal cortex These are steroid hormones involved in: • reproduction and sexual differentiation • development and growth • regulation of metabolism and nutrient supply 2 Cholesterol is the obligate precursor for steroid hormones Receptor-mediated endocytosis LDL allows circulation to cholesterol Needs LDL R to enter the cell Receptor mediatied endocytosis Target cell in the adrenal cortex once cholesterol entrs the cytoplasm can be 1) storeed in lipid doplet for later 2) biosynthesis for steroids Cholesterol goes to mitochondria Converted into pregnanolone Synthesis of steroid requires trafficking from mitochondria and ER 3 The global biosynthetic pathways All leads to steroids in adrenal cortex enzymes are highly expressed differentialyl in ovaries and testes 1. Conversion to pregnonalone throguh p450 (21C) 2. Expression of biosyntehtic pathway for adrenal steroids 4 1. P450 side chain chelate = cholesterol -> pregnenolone (this only enzyme in mitochondria) 2. Two directions of biosynthesis in cortex (androgens and mineralcorticoid) 3. Need progesterone to get to all steroids 4. 21 hydroxxylate, 11 beta hydroxylase, aldosteron synthase all -> aldosterone Androgen 11β-hydroxylase mitochondria 5 Functional Zonation of the adrenal cortex Does not have 17 alpha hydroxylase thus cannot make adrogens or glucocorticoids No aldosteron synthase androgens No 11 B hydroxylase 6 11β-hydroxylase 7 Release and transport: Cortisol: • 90 % is transported by the corticosteroid-binding globulin (CBG), transcortin • Less than 5% is free half-life of 60-90 min Helper proteins protect from degradation - long t1/2 • Plasma concentration of 5-20 µg/dL Aldosterone: • ~50 % is bound with low affinity to albumin (and CBG) • Half-life of 20 min • Plasma concentration of 5-10 ng/dL MUCH MUHC less than cortisol 1000 times less 8 Glucocorticoid receptors (cortisol) Glucocorticoid in circulation Binds to CBG and transcortin Gets to site of action Cortisol is key to glucose productio in liver All steroids are lipophylic - they diffuse Receptor for glucocorticoid is CR in cytoplasm It is inactive without CR - bound to heat shock ONce cortisol binds, Heat shock released GR Dimerizes and enters the nucleus = nuclear recptor Activated receptor bind to DNA sequences Guclocortiocoid reponsive element Transcription factor = mRNA that are key to gluconeogenesis enzymes Translated in gluconeogenic enzymes 9 Mineralocorticoid receptors (aldosterone aldosterone hsp hsp Plasma hsp ) Aldosterone has same system Enters the renal tubule cells freely (steroid) Binds minarlcorticoid recptor realease heat shock Dimerizes Activated goes to nucelus Transcription of aldosterone stuff Aldosterone rgulates salt balance It will try to retain sodium and excrete potassium Get sodium back into cell sand retaains water = increases BP These genes are key to sodiuma nd possasium transport hsp 10 Renal tubule The MR receptor has a similar affinity for aldosterone and cortisol How do renal tubule cells respond to aldosterone exclusively? Both are in circulation and can diffuse freely - how to select Remeber than cortisol is higher concentration No difference in affinity - cortisol binds and activates mineralcorticoid receptors And cause too much aldosterone effect So - high enzyme concentration of 11 B OH5D which converts cortisol -> cortisone This inactivates cortisone on MC receptor : MR MR MR Licorice inhibis MR MR 11B-OHSD Cortisone NOTE licorice is a inactivator of the 11B-OH5D enzyme so more cortisol 11 The main function of cortisol is to increase plasma glucose level ↓osteoblast ↑wakefulness modulates emotion formation IGF-1 ↑the vascular tone ↑the rate of glomerular filtration ↓fibroblast formation and collagen synthesis lipolysis storage Facilitates fetus maturation In liver Stimulates lipolysis for gluconeogeneis Braks down protein to AA for gluconeogenesis Anti-insulin effect - increase glucose in plasma Blocks translocation of GLUT 4 12 Cortisol inhibits inflammatory and immune responses: $$$$$$$ Used in orgon transplant Shut down immune system Inflammation: 1. inhibit phosphatidlcholine into acrachidonic acid: inhibits prostaglandin/ thromboxane, leukotrienes Immune: inhibits macrophages productin of 1B Dexamethasone was shown to be one of the most efficient drugs to treat patients with COVID-19, increasing the survival rate (probably linked to reducing/preventing the “cytokines storm”) . Increased survivial rate by a lot Glucocorticoids inhibit the conversion of phosphatidyl choline to arachidonic acid by inducing the production of lipocortin which inhibits phospholipase-A2 (PL-A2). They inhibit the production of inflammatory prostaglandins and thromboxanes by inhibiting cycloxygenase (COX). They inhibit the production and action of leukotrienes which are also formed from arachidonic acid by lipo-oxygenase (L-O). They block cytokine (IL-1β) production, reduce the number of circulating T cells and so reduce antibody production. Synthetic analogs of corticosteroids (prednisone) treat chronic inflammatory diseases, asthma (inhaled) Prednisone key to inflammatin and astma 13 Table summarizing cortisol effects 14 Control of cortisol synthesis and secretion by the hypothalamic-anterior pituitary axis. CRH: corticotropin-releasing hormone ACTH: adrenocorticotropin hormone Srtress produces CRH goes to anterior pituitary to stimulate ACTH Stimulates adrenal cortex and glucocorticoid How to control cotrisol - negative feedback of CRH and ACTH 16 Control of cortisol synthesis by ACTH in the Zona Fasciculata : melanocortin 2 receptor ACTH binds to G protein receptor Occcurs in zona fasciculata Activation of adenylate cyclase Activates protein kinase A - cAMP Stimulates free cholesterol release Goes to mitochondria - trasnfered to outer then inner membrane p450scc converts to pregnenloolne ACTH stimulates • hydrolysis of cholesterol esters by cholesterol esterase • transfer of cholesterol to the outer membrane of the mitochondria by a sterol transfer protein. • Transfer of cholesterol to the inner mitochondrial membrane by a steroidogenic acute regulatory (StAR) protein • P450scc that converts cholesterol to pregnenolone. 17 Aldosterone is the primary regulator of salt balance and extracellular volume Aldosterone primary regulator of salt anwa ter Retain sodium = retain water Activates gene for sodium channels When there is low BP sensed by kidney produces renin - ANGio II aldosterone and vasoconstricti on • In stress-related environment: retention of salt and water • Blockade of aldosterone secretion hyperkalemia 15 Control of aldosterone synthesis by the renin-angiotensin system: Decreaese in pressure, sodium, or increase potassium juxtaglomerular cell senses and activates renin In liver - angiotensinogen -> angiotensin i -> angiotensin II (ACE) Angiotensin II = increases BP Makes you thirsty, induces vasoconstriction, icnrease aldosterone secretion Increase potasium secretion Block with increase AMP Increase BV and stimulated through stretch in heart cGMP inhibits aldosterone synthesis (-) ANP: atrial natriuretic peptide cGMP 18 Cushing’s syndrome is due to an excess of glucocorticoids Most are due to too much glucocorticoids This is not Cushings diasease Causes of Cushing's syndrome Common (~ 99%) Exogenous therapeutic glucocorticoids Uncommon (~ <1%) - Anterior pituitary adenoma: ACTHdependent (Cushing’ disease) - Adrenal adenoma 19 Moon face, red cheeks Recedign hariline (androgens - not CUshings) 20 Clinical features of Cushing's syndrome Weight gain, central obesity Hypertension Impaired glucose tolerance/diabetes mellitus Purple striae (~60%) Osteopenia/osteoporosis (~50%) Proximal myopathy ↓osteoblast formation ↑wakefulness modulates emotion ↑the vascular tone ↑the rate of Weigh gain is weird because cortisol stimulates lipolysis glomerular its a different type of fat deposition filtration Hypertension due to cortisol increasing vascular tone Diabetes mellitus due to anti-insulic effect Purple striae due to cortisol causing decraes dibroblast and collagen = loss in elasticity Osteoporosis since cortisol negative on osteoblast ↓fibroblast formation and collagen synthesis lipolysis Facilitates fetus maturation storage Cortisol breaks down protein to amino acids = myopathy Causes of hypoadrenalism (decreased adrenal function) negatie feeedback shuts down glucocorticoids so abrupt cessatio means no more Common (~ >99%) Abrupt cessation of exogenous sources of glucocorticoids Gotta slow down with dose removal Decrease of adrenal function Abrupt sensation of glucocorticoids Rare (~ <1%) Primary adrenal insufficiency (Adrenal cortex destruction: Addison's disease) Secondary (any pituitary disease causing hypopituitarism: CRH or ACTH deficiency ) Clinical features of Addison's disease Weakness (~100%) Weight loss (~100%) Increased Pigmentation (~95%) Postural hypotension (~25%) Anorexia (~95%) 22 In Addison’s disease: high levels of ACTH and of MSH (Melanocytes Stimulating Hormone): ↑pigmentation) Increased pigmentation because ACTH is a peptide with alpha melanocyte simtulating hormone Coritcosteroids help a lot 23 Poor adrenal means not anough cortisol No negative feedback Means lots of ACTH produced and melanocyte stimulating hormone Addison's disease: loss of the cortisol negative feed-back loop leads to increased ACTH production. 24 Most common genetic diseases affecting adrenal function Most common defiicnecy Opposite as 21 Convert cortisol into cortisone So will cause excess minalcorticoid activity 11β-hydroxylase Still make androgens very well Excess androgen Salt wasting 26 11β-hydroxylase deficiency Worsk well like aldosterone 11β-hydroxylase Still produce deoxycorticosterone and deoxycortisol There is a huge accumulation Result is minalcorticoid excess - shows HTN etc. AME: apparent mineralocorticoid excess. 27 CAH: congenital adrenal hyperplasia All of this cause adrenal hyperpalsia since they keep stimulating andreogen 21-hydroxylase deficiency is the most common (salt wasting) 11-β-hydroxylase deficiency (AME)




