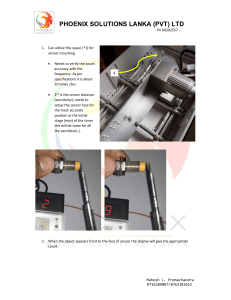
Medical Device Technical Manual: Operation & Maintenance
advertisement

Content 1 Safety Information ...................................................................... 1-1 2 Notices ........................................................................................ 2-1 2.1 2.2 2.3 2.4 2.5 2.6 2.7 Warranty Notice .....................................................................................................2-1 Copyright Notice ....................................................................................................2-1 EMC Notice.............................................................................................................2-1 MRI Notice..............................................................................................................2-1 Intended Use Notice...............................................................................................2-2 IEC Classification.....................................................................................................2-2 Declaration of Conformity Notice ..........................................................................2-2 3 Equipment Symbols ..................................................................... 3-1 4 Introduction ................................................................................ 4-1 4.1 4.2 General description ................................................................................................4-1 Abbreviations and Definitions ................................................................................4-2 5 Theory of Operation .................................................................... 5-1 5.1 Pneumatic System ..................................................................................................5-1 5.1.1 Pneumatic Theory ..........................................................................................5-1 5.1.2 Main Pneumatic Assemblies Overview ..........................................................5-3 5.2 Electrical System.................................................................................................. 5-12 5.2.1 Electrical Box ............................................................................................... 5-12 5.2.2 Expiratory Valve Heating Module ................................................................ 5-13 5.2.3 Rear Panel View........................................................................................... 5-14 5.2.4 Display Inside View...................................................................................... 5-15 5.2.5 ARM platform circuit board......................................................................... 5-16 5.2.6 Display interface board ............................................................................... 5-17 5.2.7 Interface Board ............................................................................................ 5-18 5.2.8 Mother Board .............................................................................................. 5-19 5.2.9 Core Board................................................................................................... 5-21 5.2.10 Main Control Board ..................................................................................... 5-22 5.2.11 Power Supply Board .................................................................................... 5-23 5.2.12 System Wiring Diagram ............................................................................... 5-24 5.3 Software System .................................................................................................. 5-30 5.3.1 Software Units and Their Relationship ........................................................ 5-30 5.3.2 Structure of the Software System ............................................................... 5-31 5.3.3 Hardware and Software Interface ............................................................... 5-32 6 Disassemble and Functional Testing ............................................ 6-1 6.1 General ...................................................................................................................6-1 i 6.2 Main Assemblies Disassemble ...............................................................................6-1 6.2.1 Disassembling of the Display from the Host ..................................................6-1 6.2.2 Disassembling of the Host from the Trolley ...................................................6-2 6.2.3 Disassembling of the Arm Assembly from the Host .......................................6-3 6.2.4 Disassembling of the Humidification Assembly from the Host ......................6-4 6.3 Disassembling of Maintenance Spare Parts ...........................................................6-5 6.3.1 Disassembling of Oxygen Sensor ....................................................................6-5 6.3.2 Disassembling of Pressure Sensor Board .......................................................6-6 6.3.3 Disassembling of Supply O2 Assembly............................................................6-7 6.3.4 Disassembling of TSI Mass Flow Sensor .........................................................6-9 6.3.5 Disassembling of Differential Pressure Transmitter .................................... 6-10 6.3.6 Disassembling of Diaphragm and Scale Board ............................................ 6-12 6.3.7 Disassembling of Core Board and Main Control Board ............................... 6-13 6.3.8 Disassembling of Components in the Display ............................................. 6-14 6.3.9 Disassembling of Electric Panel Assembly................................................... 6-16 6.3.10 Disassembling of One-way Diaphragm........................................................ 6-17 6.3.11 Disassembling of Fan Filter Cotton .............................................................. 6-18 6.3.12 Disassembling of Filter Element of Gas Inlet............................................... 6-19 6.3.13 Disassembling of Filter (Part No.:130003930)............................................. 6-20 6.4 Functional Testing ............................................................................................... 6-21 7 User Maintenance ....................................................................... 7-1 7.1 7.2 7.3 7.4 7.5 7.6 ii Cleaning and Disinfection .......................................................................................7-2 7.1.1 Cleaning and Disinfecting Agents/ Autoclaving..............................................7-3 7.1.2 Cleaning and Disinfection Methods ...............................................................7-3 7.1.3 Cleaning and Disinfection of Components .....................................................7-4 Regular Maintenance .............................................................................................7-6 7.2.1 Maintenance Principles ..................................................................................7-6 7.2.2 User Maintenance ..........................................................................................7-6 7.2.3 Service Life of Product/Accessories................................................................7-7 Maintenance in Operation and Transportation......................................................7-8 7.3.1 Transportation ................................................................................................7-8 7.3.2 Storage ...........................................................................................................7-9 Fuse Replacement ..................................................................................................7-9 Battery Maintenance........................................................................................... 7-10 7.5.1 Battery Specification ................................................................................... 7-10 7.5.2 Precautions.................................................................................................. 7-11 7.5.3 Battery Storage ............................................................................................ 7-11 7.5.4 Battery Replacement ................................................................................... 7-11 7.5.5 Battery Charging and Calibration ................................................................ 7-12 Oxygen Sensor ..................................................................................................... 7-12 7.6.1 Oxygen Sensor Replacement ....................................................................... 7-12 7.6.2 Oxygen Sensor Calibration .......................................................................... 7-13 7.6.3 Technical Specifications of Oxygen Sensor .................................................. 7-13 7.6.4 Oxygen Sensor Maintenance....................................................................... 7-14 7.7 Paramagnetic Oxygen Sensor (optional) ............................................................. 7-14 7.7.1 Paramagnetic Oxygen Sensor Calibration ................................................... 7-15 7.7.2 Technical Specifications of Paramagnetic Oxygen Sensor ........................... 7-15 7.8 Disposal ............................................................................................................... 7-16 7.9 Manufacturing techniques and materials: .......................................................... 7-16 7.10 Free Obligations: ................................................................................................. 7-17 7.11 Security, reliability and operating condition: ...................................................... 7-17 7.12 Return.................................................................................................................. 7-17 8 Pre-Use Test ................................................................................ 8-1 8.1 8.2 Pre-use test procedure ...........................................................................................8-1 Pre-use test failure analysis ....................................................................................8-2 9 Network ...................................................................................... 9-1 9.1 9.2 9.3 9.4 9.5 9.6 10 Overview ................................................................................................................9-1 What is Exported? ..................................................................................................9-1 Establishing a Connection ......................................................................................9-1 Interface Instructions .............................................................................................9-2 Transmits and Receives Data ..................................................................................9-4 Troubleshooting .....................................................................................................9-5 Service Menu ....................................................................... 10-1 10.1 Calibration ........................................................................................................... 10-2 10.1.1 Calibration operation guidance ................................................................... 10-2 10.1.2 Calibration failure analysis .......................................................................... 10-8 10.2 Event/Alarm Log .................................................................................................. 10-9 10.3 Machine Information ........................................................................................ 10-10 10.4 Language ........................................................................................................... 10-10 10.5 Test Page............................................................................................................ 10-10 10.5.1 Demo ......................................................................................................... 10-11 10.5.2 Vlt. Monitor ............................................................................................... 10-11 10.5.3 Schematic .................................................................................................. 10-12 10.5.4 Cali. Data ................................................................................................... 10-13 10.5.5 Service Timer ............................................................................................. 10-13 10.5.6 Error Code ................................................................................................. 10-14 10.5.7 PT100 Cali. ................................................................................................. 10-14 10.6 Update ............................................................................................................... 10-15 10.7 Optional............................................................................................................. 10-16 11 Troubleshooting................................................................... 11-1 11.1 Technical Error ..................................................................................................... 11-1 11.1.1 Technical Test Error Code ............................................................................ 11-1 11.1.2 Other Errors................................................................................................. 11-4 iii 11.1.3 Service Tools ................................................................................................ 11-4 12 Appendix A Contact & Ordering Information ....................... 12-1 13 Appendix B Diagrams and Schematics.................................. 13-1 14 Appendix C Specifications .................................................... 14-1 15 Appendix D Spare parts and configurations ......................... 15-1 iv 1 Safety Information 1 Safety Information The operator of this ventilator must recognize their full responsibility for choosing appropriate ventilation settings to ensure proper ventilation and patient safety. The responsibility for the selection of the appropriate level of patient monitoring depends solely on the equipment operator. All the monitoring information is for reference only; it should not be used as the sole basis for therapeutic or diagnostic decisions. Whenever a patient is connected to the ventilator, constant attention by qualified medical personnel is required in order to provide immediate corrective action in case of a malfunction and alarm occurrence. Terms This manual uses three special indicators to convey information of a specific nature. They include: WARNING: Identify conditions or practices that could result in serious adverse reactions or potential safety hazards. CAUTION: Identify conditions or practices that could result in damage to the ventilator or other equipment NOTE: Identify supplemental information to help you better understand how the ventilator works. WARNING: Do not use the system until you have read and understood this manual including: All connections of the system All warnings and cautions Operation procedure of each and every component of the system Test procedure of each and every component of the system WARNING: The VG70 Ventilator System is intended for use by authorized and trained medical personnel only. WARNING: The users must familiarize themselves with the operation and use of this machine prior to first clinical use with a patient. 1-1 WARNING: To ensure proper servicing and avoid the possibility of physical injury, only qualified personnel should attempt to service or make authorized modifications to the ventilator. WARNING: An authorized service engineer must first install the ventilator and run Aeonmed’s installation procedure, which includes calibration of various system components, before you connect a patient to the ventilator. WARNING: If a fault is detected in the VG70 ventilator so that its life support functions are no longer assured: start ventilation using an independent ventilation device (resuscitation bag) without delay, if necessary with PEEP and/or increased inspiratory O2 concentration. WARNING: Before activating any part of the ventilator, be sure to check the equipment for proper operation and, if appropriate, run PUT (pre-use test) as described in this manual, WARNING: The VG70 ventilator is not intended to be a comprehensive monitoring device and does not activate alarms for all types of dangerous conditions for patients on life-support equipment. WARNING: Patients on life-support equipment must be appropriately monitored by competent medical personnel and suitable monitoring devices at all times. WARNING: An alternative source of ventilation, such as manual respiratory equipment, should always be available when using the VG70 ventilator. WARNING: Do not connect inspiratory or expiratory circuits to the exhaust port. WARNING: Ensure that inspiratory and expiratory circuits are connected to the correct port before operation of equipment. WARNING: The expiratory gas pathway may become contaminated with body fluids or expired gases during normal use, and the inspiratory gas pathway may become contaminated during fault condition, such as occlusion, breath hoses disconnection. 1-2 1 Safety Information WARNING: Disposable breathing hoses shall not be reused. Reuse of the single use hoses can cause cross infection. WARNING: Assure that hoses used have the appropriate resistance and compliance to ensure proper therapy. WARNING: Do not disconnect the cable between the Main Control Unit and the GUI screen while Ventilator is operating. WARNING: The ventilator must not be connected to any anti-static or electrically conductive hoses, tubing or conduit WARNING: Adding attachments or other components or sub-assemblies to the ventilator breathing system can change the pressure gradient across the ventilator breathing system and that such changes to the ventilation breathing system can affect the ventilator performance. WARNING: Expiratory module is heated; use caution to avoid burns. WARNING: Use caution when handling flammable or fragile components. WARNING: Do not place containers of liquids (such as humidifier water reservoirs) on top of or above ventilator. Liquids getting into the ventilator can cause equipment malfunction with the risk of patient injury. WARNING: To avoid risk of electric shock, this equipment must only be connected to a supply main with protective earth. CAUTION: The breathing circuit must not be installed whenever the VG70 powers up and whenever a pre-use test is performed. CAUTION: If the system test fails, do not use the system. Attempt to troubleshoot and fix the failure. If you are unable to fix the device, ask an authorized service representative to repair the device. 1-3 CAUTION: Check the ventilator periodically as outlined in this manual; do not use if defective. Immediately replace parts that are broken, missing, obviously worn, distorted, or contaminated. CAUTION: Do not put ventilator into service until the patient setup is complete. CAUTION: Measurements can be affected by mobile and RF communications equipment. CAUTION: Do not use oxygen hoses that are worn, frayed, or contaminated by combustible materials such as grease or oils. Textiles, oils, and other combustibles are easily ignited and burn with great intensity in air enriched with oxygen. CAUTION: Follow your hospital infection control guidelines for handling infectious material. Aeonmed recognizes that cleaning, sterilization, sanitation, and disinfection practices vary widely among health care institutions. It is not possible for Aeonmed to specify or require specific practices that will meet all needs, or to be responsible for the effectiveness of cleaning, sterilization, and other practices carried out in the patient care setting. CAUTION: Equipment not suitable for use in the presence of a Flammable Anesthetic mixture with Air or with Oxygen or Nitrous Oxide. CAUTION: To avoid an electrical shock hazard while servicing the ventilator, be sure to remove all power to the ventilator by disconnecting the power source and turning off all ventilator power switches. CAUTION: To avoid a fire hazard, keep matches, lighted cigarettes, and all other sources of ignition (e.g., flammable anesthetics and/or heaters) away from the ventilator and oxygen hoses. CAUTION: In case of fire or a burning odor, immediately disconnect the ventilator from the oxygen supply, facility power and backup power source. CAUTION: During operation, do not block: Speaker Holes, Exhaust Port, Air Inlet or Cooling Fan. 1-4 1 Safety Information CAUTION: Do not use the VG70 Ventilator in a MRI environment. CAUTION: The ventilator shall not be used in a hyperbaric chamber. CAUTION: The ventilator shall not be used with helium or mixtures with helium. CAUTION: Tip over hazard; use care when moving ventilator mounted to cart as device could tip over leading to injury or damage of equipment. CAUTION: Do not use sharp objects to make selections on the LCD touch screen or panel. CAUTION: Do not connect a VGA or USB interface while the system is in service CAUTION: The Network interface connection is for authorized service only. CAUTION: Batteries should be removed if equipment will not be in service for more than 6 months. CAUTION: Do not immerse the oxygen sensor or the connector in any type of liquid. CAUTION: When ventilator is exposed to conditions outside the specified operating environment, allow 24 hours in normal environment before using. CAUTION: Storage environment: -20℃~+60℃ and ≤95%RH. CAUTION: Operating environment: 5℃~40℃ and 5%RH~95%RH. CAUTION: Do not connect items that are not specified as part of the system. CAUTION: The auxiliary outlet is only for the recommended humidifier; do not connect to any other equipment or an additional multiple socket outlets. CAUTION: When using a humidifier, user should frequently check the water trap and look for water in the hose. If water is found in the hose, this water should be removed. 1-5 Also, it is important the water trap is positioned in a way such that it is lower than the patient tubes. CAUTION: Connecting electrical equipment to auxiliary outlet effectively leads to creating a medical equipment system, and can result in a reduced level of safety, make sure the ME SYSTEM comply with requirements of IEC 60601-1:2005. The user who connects is responsible for the standard for the requirements applicable to the medical equipment system. CAUTION: USA Federal law restricts this device for sale by or on the order of a physician. NOTE: The user of this product shall have sole responsibility for any ventilator malfunction due to operation or maintenance performed by anyone not trained by Aeonmed. NOTE: Usage of a filter on the expiratory side will increase the resistance of the patient circuit. NOTE: In non-invasive (NIV) ventilation, the exhaled volume of the patient can differ from the measured exhaled volume due to leaks around the mask NOTE: Do not sterilize or immerse the Mainstream CO2 Adapter in any fluids. NOTE: All parts of the ventilator system are suitable for use within the patient environment. NOTE: All gas volume, flow, and leakage specifications in this manual are expressed at STPD (standard temperature and pressure dry), except when specified with another condition. 1-6 2 Notices 2 Notices 2.1 Warranty Notice Do not make any service repairs on this equipment during the states warranty period. Any unauthorized work immediately voids the warranty. Aeonmed will not be liable for any repairs attempted by the owner. Any such attempted repairs other than specified no warranty repairs void the warranty. 2.2 Copyright Notice Copyright Notice: Aeonmed. This work is protected the copyright law and is the sole property of the Company. No part of this document permitted to be copied or otherwise reproduced, translated into other languages, or stored in any electronic information retrieval system, without the prior written consent of the Company. 2.3 EMC Notice This equipment can radiate radio frequency energy ,as well can be interfered by electromagnetic If not installed and used in accordance with the instructions mentioned in the manual, electromagnetic interference may result. The equipment has been tested and found to comply with the limits set forth in EN60601-1-2 for Medical Products. These limits provide reasonable protection against electromagnetic interference when operated in the intended use environments described in this manual. The ventilator has been tested to conform to the following specifications: MIL-STD-461D: 1993, MIL-STD-462D:1993, EN 55011:2007+A2:2007, IEC 1000-4-2: 1994, IEC 1000-4-3:1994 IEC 1000-4-4:1994, IEC 1000-4-5:1994, QUASI-STATIC:1993 This ventilator is also designed and manufactured to comply with the safety requirements of IEC 60601-1, IEC 60601-2-12, CAN/CSA C22.2 No. 601.1-M90, and UL 60601-1. 2.4 MRI Notice This equipment contains electromagnetic components whose operation can be affected by intense electromagnetic fields. Do not operate the ventilator in a MRI environment or in 2-1 the vicinity of high-frequency surgical diathermy equipment, defibrillators, or short-wave therapy equipment. Electromagnetic interference could disrupt the operation of the ventilator. 2.5 Intended Use Notice The VG70 Critical Care Ventilator is intended for patients ranging from pediatric to adult, and for use in a wide variety of clinical conditions. Specifically, the VG70 Critical Care Ventilator is applicable for adult and pediatric patients weighing at least 5 kg for adult and 3 Kg for child (7lbs.), who require the following types of ventilatory support: Positive Pressure Ventilation, delivered invasively (by ET or Tracheotomy tube) or non-invasively (by mask) via Assist/Control, SIMV, CPAP and other modes of ventilation. The VG70 Critical Care Ventilator is intended for use in hospital and hospital-type facilities. It may be used during intra-hospital transport provided that electrical power and compressed gas are supplied. WARNING: The VG70 Ventilator is not designed for use in an MRI environment. Do not use the VG70 Ventilator near an MRI machine or injury or equipment damage could result. WARNING: The user of the Ventilator must be professional and trained. 2.6 IEC Classification Type of Equipment: Medical Equipment, Class I, Type B. Adult/Pediatric Lung Ventilator 2.7 Declaration of Conformity Notice This medical equipment complies with the Medical Device Directive, 93/42/EEC, and the following Technical Standards, to which Conformity is declared: IEC 60601-1: The device classification is: Class I, Type B applied part (ventilator breathing circuit and mask), type BF applied part (CO2 module), ordinary enclosed equipment without protection against ingress of liquids, continuous operation ISO 80601-2-12 IEC 60601-1 2-2 3 Equipment Symbols 3 Equipment Symbols Warnings and Cautions indicate all the possible dangers in case of violation of the stipulations in this manual. Refer to and follow them. Warnings Indicate potential hazards to operators or patients. Cautions Indicate potential damage to equipment. Instead of illustrations, symbols may be utilized. Not all of these symbols may necessarily appear on the equipment or in this User manual. The symbols include: On (Power) Protection Class Type B Off (Power) Protection Class Type BF Follow operating instructions Warning & Caution Protective earth ground Dangerous voltage EQUIPOTENTIAL connection loudspeaker Lock Manufacturer Unlock Date of production Inspiratory hold Serial Number 3-1 Nebulization Expiratory hold Intelligent increase of oxygen Manual inspiration Standby Waveform freeze AC power Internal Battery USB device Refer to documentation Prompt message Already online Flow trigger Pressure trigger Adult Manual trigger NIV modes Child Main Menu Invasive modes neonate Alarm Silence Key Do not reuse Disposal of Waste The system, with this label under the stipulations in the operating manual, complies with the requirements related from 93/42/EEC. 0123 is the certificate number to certify authorizations 3-2 int. 4 Introduction 4 Introduction 4.1 General description The VG70 Critical Care Ventilator System consists of two required components: a Main Control Unit and a Graphical User Interface (GUI). Optional Components available for the VG70 Critical Care Ventilator system are: Ventilator Cart, Gas Cylinder Transport Cart, Patient Circuit Positioning Arm, and Hose Assemblies for O2, CO2 monitoring subsystem, etc. 1 Main Control Unit 2 Humidifier 3 Patient Circuit 4 Water Trap 5 Nebulizer Tube 6 Y-piece 7 Test Lung 8 Mask 9 Connector 10 Nebulizer Connector 11 Patient Circuit Positioning Arm 4-1 4.2 Abbreviations and Definitions (S) Means Set Value (M) Means Measured Value CPAP Continuous Positive Airway Pressure (Set value, hereinafter “S”) F Breath rate (frequency) in bpm, i.e. ventilation times per minute (S) Patient’s fspont spontaneous respiratory frequency (Measured value, hereinafter “M”) Total breath rate, i.e. the sum of breath rate f and spontaneous breath ftotal rate fspont(M) O2 Inspiratory O2 concentration (S & M) I:E The ratio of Inspiration to Expiration (M) MV Expiratory minute volume (M) MVspont Spontaneously breathed minute volume(M) MVleak Leakage minute volume (M) Paw Patient airway pressure (M) Positive End-Expiratory Pressure, which can improve the patient’s PEEP oxygenation (S & M) PEEPi Intrinsic Positive End-Expiratory Pressure (M) Pinsp Upper pressure level in PCV mode (S) Mean airway pressure. This value is updated at the end of the last Pmean 4-2 respiratory cycle, hence, is a continuous average (M) Ppeak Airway pressure peak value during one ventilatory cycle (M) Pplat End-inspiratory airway pressure (M) Pmin Minimum airway pressure (M) Psens Pressure sensitivity (S) 4 Introduction Psupp Pressure support (S) Phigh Upper pressure level in BIVENT and APRV (S) Plow Lower pressure level in BIVENT and APRV (S) T Imax Maximum inspiratory time (S) Tinsp Inspiratory Time (S) Inspiratory Pause Time, to increase the inspiratory time to improve the T pause patient’s oxygenation (S) Trigger by flow rate (S) VT Tidal volume of mechanical ventilation (S) VTE Expiratory tidal volume (M) VTI Inspiratory tidal volume (M) Esens Expiratory trigger sensitivity (S) ETCO2 End-expiratory CO2 concentration (M) WOB Work of breathing (M) R*C Time constant (M) Leak% Leakage percentage (M) Cdyn Dynamic compliance (M) Cstatic Static compliance (M) Rinsp Inspiratoryresistance (M) Rexp Expiratory resistance (M) Elastic Elastic resistance (M) IP21 Solid particle protection level 2; Liquid ingress protection level 1 4-3 5 Theory of Operation 5 Theory of Operation 5.1 Pneumatic System 5.1.1 Pneumatic Theory 5-1 Figure 5-1 Pneumatic Theory 5-2 5 Theory of Operation 5.1.2 Main Pneumatic Assemblies Overview 4 1 2 3 Figure 5-2 Main Airway Assemblies sketch view 1 Turbine driver 2 Expiratory valve module 3 Gas circuit module 4 Electrical box 5-3 Pneumatic Connecting Diagram 4 7 Figure 5-3 Pneumatic Connecting Diagram PU tube φ6 Silica gel tube φ2x1.5 Silica gel tube φ1x1 5-4 1 Oxygen inlet module 2 Oxygen supply module 3 Inferoanterior shell components 4 Differential pressure transmitter 5 MMB 6 Safety inspiration module 7 Exhalation valve components 5 Theory of Operation Gas Circuit Module 1 2 3 5 4 Figure 5-4 Gas circuit module 1 Differential pressure transmitter 2 Interface board 4 Pressure sensor board 5 Fan 3 Inspiratory flow sensor 5-5 Oxygen Inlet Module Overview Figure 5-5 Supply Oxygen Module item Description Gas flow direction 5-6 1 Oxygen connector, connecting with oxygen supplies. 2 Low flow oxygen connector, connecting with low flow oxygen supplies. 3 Inlet filter, filtering impurities from the oxygen. 4 Pressure sensor, monitoring the pressure of high-pressure oxygen gas. 5 one-way valve 6 Regulator valve, regulating gas pressure to 0.28MPa 5 Theory of Operation 7 Connector, connecting with oxygen mixer module, supply low flow oxygen 8 Connector, connecting with oxygen mixer module, supply high pressure oxygen 9 Inlet filter Supply Oxygen Module Overview Figure 5-6 Oxygen Mixer Module Item Description Gas flow direction 1 Connector, connecting with connector 7(low flow oxygen) in Figure 5-5 Supply Oxygen Module 2 Connector, connecting with Turbine and Inspiratory Control Module 3 Solenoid valve V1-V4, Flow 30L/min 4 Connector, connecting with connector 8(high pressure oxygen) in Figure 5-5 Supply Oxygen Module 5 Connector, connecting with nebulizer pipe 6 Solenoid Valve V9, controlling whether affording the oxygen to nebulizer 5-7 7 Solenoid valve V7-V8, Flow 4L/min 8 Solenoid valve V5-V6, Flow 12L/min Turbine and Inspiratory Control Module Overview Figure 5-7 Turbine and Inspiratory Control Module 5-8 5 Theory of Operation Item Description Gas flow direction 1 Inspiratory Valve, control of inspiratory flow and pressure 2 Connector, connecting with the pressure sensor of mother board 3 Air intake, including air filter 4 Mixing chamber, mixing oxygen and air 5 Muffler 6 Turbine 7 Radiator 8 Flow sensor, monitoring the volume of the gas 9 Muffler 10 Connector, connecting with safe inspiratory module Safety Inspiratory Module Overview 5-9 Figure 5-8 Safety Inspiratory Module Item Description Gas flow direction 5-10 1 Inspiratory connector, connecting with silica gel tube for patient 2 Oxygen sensor, monitoring the oxygen density of mixed gas 3 Relief valve 4 Emergency air inlet 5 Connector, connecting with flow sensor 6 One-way valve 7 Connector, connecting with the pressure sensor of mother board 5 Theory of Operation Expiratory Module Overview 1 2 Figure 5-9 3 4 Figure 5-10 Item Description Movement direction 1 Connector, connecting with pressure sensor of mother board. 2 Driving valve 3 Press the lock nut of the expiration valve, unlock, then pull out the component. 4 Water trap 5-11 5.2 Electrical System 5.2.1 Electrical Box 3 2 1 4 1 5 6 7 Figure 5-11 5-12 1 Core board 2 Main control board 3 Battery box 4 Battery 5 Power supply board 6 Mother board 7 Switch power 5 Theory of Operation 5.2.2 Expiratory Valve Heating Module 1 2 Figure 5-12 1 Temperature sensor 2 Heating resistor 5-13 5.2.3 Rear Panel View Figure 5-13 1 CO2 module connector 2 Nurse call connector 3 DC power fuse 4 Air inlet 5 Oxygen inlet 6 Fan 7 Equipotential terminal 8 DC power input port 9 Power switch 10 AC power input socket 11 Battery cover 5-14 5 Theory of Operation 5.2.4 Display Inside View Figure 5-14 1 Indicator light board 2 Speaker 3 Mute key board 4 Encoder board 5 Display cable 6 Display platform circuit board 7 Display connector 8 VGA connector 9 USB connector 10 NET connector 11 Touch screen controller 12 Alarm LED board 5-15 5.2.5 ARM platform circuit board Figure 5-15 5-16 1 Power supply indicate lamp board connector 2 Backlight cable connector 3 Mute button board connector 4 Encoder board connector 5 Display cable connector 6 Interface board connector 7 Touch screen control board connector 8 Alarm light board connector 9 LVDS cable connector 10 Speaker cable connector 5 Theory of Operation 5.2.6 Display interface board Figure 5-16 1 CN1 interface (Connect Display platform circuit board) 2 VGA interface 3 USB 2 interface 4 USB 1 interface 5 NET interface 5-17 5.2.7 Interface Board Figure 5-17 5-18 1 Signal 2 Motor controller 3 Insp. valve 4 Turbine 5 Fan 6 Turbo tempeture sensor 7 PEEP valve 8 Expe. valve heating 9 Expe. flow sensor board 10 O2 sensor 11 Expe. valve tempeture sensor 12 TSI sensor 13 Power in 5 Theory of Operation 5.2.8 Mother Board Figure 5-18 Front of mother board Figure 5-19 Back of mother board 1 Core board slot 2 Reserved 3 Analogy slot 4 Lithium-ion battery 1 connector 5 Lithium-ion battery 2 connector 5-19 6 PSB slot 7 Main switch connector 8 24VDC input connector 9 DC power input connector 10 Display connector 11 Reserved 12 Reserved 13 Motor controller connector 14 Reserved 15 O2 supply pressure sensor 16 Paramagnetic oxygen sensor connector (optional) 17 Interface board signal line connector 18, 19 20 5-20 Solenoids cable connector Interface board power line connector 5 Theory of Operation 5.2.9 Core Board Figure 5-20 1 Mother board connecting connector 2 DSP_JTAG 5-21 5.2.10 Main Control Board Figure 5-21 5-22 1 Mother board connecting connector 2 CO2 module connector 3 Nurse call connector 5 Theory of Operation 5.2.11 Power Supply Board 3 Figure 5-22 1 Mother board connecting connector 5-23 5.2.12System Wiring Diagram BOM size and diagram: No. BOM size 1 122007322 Fan cable 2 122007320 Interface board signal cable 3 122007321 PT100 signal cable 4 122008090 Expiration valve heating component 5 122008011 Magnetic base component 5-24 Name Diagram 5 Theory of Operation No. BOM size Name 6 122007331 Oxygen supply pressure cable 7 122007327 Expiration flow sensor cable 8 122007329 TSI sensor cable (analog) 9 122007330 TSI sensor cable (digital) 10 122007328 Oxygen sensor signal cable Diagram 5-25 No. BOM size 11 122007315 Valve terminal drive cable (30L) 12 122007316 Valve terminal drive cable (12L) 13 122007317 Valve terminal drive cable(4L) 14 122007319 Interface board power cable 15 122007478 Turbine driver component 5-26 Name Diagram 5 Theory of Operation No. BOM size Name 16 122007336 Switch power output cable 17 122012312 W0056 cable 18 122007486 Power lamp component 19 122007484 Speaker component 20 122007483 Mute button component 21 122012310 W0054 cable Diagram 5-27 No. BOM size 22 122007455 Switch mounting plate component 23 122007463 Inspiration control valve 24 122007482 Encoder component 25 122007485 Alarm lamp component 26 122007503 Copper woven mesh cable 5-28 Name Diagram 5 Theory of Operation No. BOM size Name 27 122012313 W0057 cable 28 122012311 W0055 cable 29 122009449 W0001 cable (optional) 30 122001124 W1 cable Diagram 5-29 5.3 Software System 5.3.1 Software Units and Their Relationship User Interface Software Unit bears the responsibility of user interface of the whole software system. It includes operation interface for the users, graphic interface for showing information of the device, communication with other units, etc. Breathing Delivery Software Unit bears the responsibility of ventilation control. Power Supply Software Unit bears the responsibility of power management. The relationship between these three units is as following: User Interface SW Unit Breathing Delivery SW Unit Power Supply SW Unit Figure 5-23 Software Units of NBP System In above figure, the orientation of arrow points to information is flowing to where. Furthermore, a bidirectional arrow means communication between SW units is bidirectional. 5-30 5 Theory of Operation 5.3.2 Structure of the Software System Figure 5-24 Structure of Software Units 5-31 5.3.3 Hardware and Software Interface Figure 5-25 Interface between Software and Hardware System 5-32 6 Disassemble and Functional Testing 6 Disassemble and Functional Testing 6.1 General The ventilator is packaged with two cartons. One carton filled with cart. Another carton filled with ventilator main units, display, and accessories crate. Accessories crate including standard accessories and optional accessories. Standard accessories are divided into European standard and American standard, the optional accessories is changed based on order. Details please refer to Appendix D configuration List. 6.2 Main Assemblies Disassemble 6.2.1 Disassembling of the Display from the Host Figure 6-1 To separate the display from the host: pull out the display (1) along the guide (2). 6-1 6.2.2 Disassembling of the Host from the Trolley Figure 6-2 To separate the host from the trolley a. Remove the screw (2); b. Remove the host (1) along “ c. Remove the host installing piece (3) from the tray connector (4). d. Lift up the host (1); ” direction; NOTE: When installing the host to the trolley, please align the host installing piece with the tray connector. 6-2 6 Disassemble and Functional Testing 6.2.3 Disassembling of the Arm Assembly from the Host Figure 6-3 To separate the arm assembly from the Main Control Unit a. Pull out the knob plunger(1); b. Remove the arm assembly (3) from the handrail (2). 6-3 6.2.4 Disassembling of the Humidification Assembly from the Host Figure 6-4 To separate the humidification assembly from the host: remove the humidification assembly (1) from the guide-rail (2) along “ 6-4 ” direction. 6 Disassemble and Functional Testing 6.3 Disassembling of Maintenance Spare Parts 6.3.1 Disassembling of Oxygen Sensor Figure 6-5 To separate the oxygen sensor from the host: a. Pull out oxygen sensor cover(1); b. Remove the oxygen sensor(2). 6-5 6.3.2 Disassembling of Pressure Sensor Board Figure 6-6 To remove the pressure sensor board from the host: a. Remove the six screws (2) (Cross recessed Pan head screw M4x8); b. Move the side panel(1) to a side; c. Disconnect the cable connector of the pressure sensor; d. Remove the two screws (3) (Cross recessed Pan head screw M3x6); e. Remove the pressure sensor board (4). Test: When you replaced the oxygen pressure sensor, turn on the machine to check if the oxygen pressure value is back to normal. 6-6 6 Disassemble and Functional Testing 6.3.3 Disassembling of Supply O2 Assembly 1 2 Figure 6-7 a. Remove the screws (Hexagon socket head cap screws M4×16) of the upper cover, and remove the upper cover (1); b. Remove the inlet link block of the oxygen inlet module, and remove the filter cover of the air inlet. c. Remove the screws (Cross recessed Pan head screw M3x6) of the lower rear cover, and remove the lower rear cover (2); 1 Figure 6-8 d. Remove the screws of the electrical box, pull out the three connectors(2, 3 4) of supply O2 module from the mother board of the electrical box (5), and then remove the electrical box (1); 6-7 1 1 2 Figure 6-9 e. Remove the four screws (1) (Cross recessed Pan head screw M3x25); f. Remove supply O2 module (2). 6-8 6 Disassemble and Functional Testing 6.3.4 Disassembling of TSI Mass Flow Sensor Figure 6-10 To remove TSI mass flow sensor from the host: a. Remove the six screws (2) (Cross recessed Pan head screw M4x8); b. Move the right cover to a side(1); c. Disconnect the two cables of TSI mass flow sensor; d. Drag out the TSI mass flow sensor along “ ” direction (3). Test: When the TSI mass flow sensor is replaced, the inspiratory valve must be calibrated. Please refer to Chapter 10.1. 6-9 6.3.5 Disassembling of Differential Pressure Transmitter 3 2 1 Figure 6-11 To remove the differential pressure transmitter from the host: a. Remove the handle (1), and then remove the host from Cart assembly; b. Remove screws, and then remove the two side covers (2); c. Remove Head cover components (3); 6-10 6 Disassemble and Functional Testing 2 1 Figure 6-12 d. Remove the Electrical box components backwards to the right place (1); e. Remove the differential pressure transmitter (2). Test: When the differential pressure transmitter is replaced, the inspiratory valve must be calibrated. Please refer to Chapter 8 and Chapter 10.1. 6-11 6.3.6 Disassembling of Diaphragm and Scale Board Figure 6-13 Figure 6-14 To remove the diaphragm and the scale board from expiratory module assembly: a. Press the button(1 in Figure 6-13); b. Pull out the expiratory valve core component (2 in Figure 6-13); c. Remove the end cover (1 in Figure 6-14) and the gasket (2 in Figure 6-14); d. Remove the scale board (3 in Figure 6-14) and the diaphragm (4 in Figure 6-14). Test: When the diaphragm and scale board are replaced, the inspiratory valve must be calibrated. Please refer to the Chapter 10.1; Also perform a pre-use test and verify all test pass Refer to Chapter 8. 6-12 6 Disassemble and Functional Testing 6.3.7 Disassembling of Core Board and Main Control Board Figure 6-15 Remove of the core board and control board: a. Remove the four screws(1) (Cross recessed Pan head screws M3×6); b. Remove the electric board cover (2); 6-13 c. Pull out the core board (3); d. Pull out the main control board (4). Test: when you replaced the core board and main control board. Cut off the battery, connect the alternating current, and check if the machine operates normally. 6.3.8 Disassembling of Components in the Display a. Remove the four screws (1) (Cross recessed countersunk head screw M4×8), take off the rear shell hanger and hanging block interface board (Figure 6-16); Figure 6-16 b. Pry the back-shell of display open with the tool. You can use the tool to pry the black circle signed places (Figure 6-17). Figure 6-17 6-14 6 Disassemble and Functional Testing c. Open the back-shell slowly and remove the back-shell. d. Remove the six screws (2) (Cross recessed Pan head screws M3×6), and then remove the shielding mask (1) (Figure 6-18); Figure 6-18 e. The components in the display can be easy to be taken away. 6-15 6.3.9 Disassembling of Electric Panel Assembly Figure 6-19 a. Remove the four screws (1) (Cross recessed Pan head screws M3×6); b. Remove the electric panel cover (2); c. Remove the power supply board component (3). Test: When any circuit board has been removed and or replaced, you must perform ad calibration on all sensors and perform a pre-use check prior to use. Please refer to Chapter 8 and Chapter 10.1. 6-16 6 Disassemble and Functional Testing 6.3.10 Disassembling of One-way Diaphragm Figure 6-20 To remove the one-way diaphragm: a) Remove the expiratory valve core component; b) Remove the one-way valve core component (1); c) Remove one-way diaphragm(2); Test: When the one-way diaphragm has been replaced, the inspiratory valve must be calibrated. Please refer to chapter 8 and chapter 10.1. 6-17 6.3.11 Disassembling of Fan Filter Cotton Figure 6-21 To remove the fan filter cotton: 6-18 a. Remove the fan filter cotton cover(2); b. Remove the fan filter cotton(1). 6 Disassemble and Functional Testing 6.3.12 Disassembling of Filter Element of Gas Inlet Figure 6-22 To remove filter element of gas inlet: a. Remove the filter cover(1); b. Remove the filter support sleeve(2); c. Remove the filter element of gas inlet(3). 6-19 6.3.13 Disassembling of Filter (Part No.:130003930) Figure 6-23 To remove the filter: 6-20 a. Remove the two screws (1) (Hexagon socket head cap screws M4×10); b. Remove the oxygen inlet connector(2); c. Remove the filter (3). 6 Disassemble and Functional Testing 6.4 Functional Testing Note: Before the functional testing you need to set ventilator in standard states. Ventilator standard working states: Breathing patterns: A / C (VCV); Inspiratory time: 1 seconds; Breath-hold time: 0 seconds; Respiratory rate: 20 beats/min; Tidal volume: 400 ml; The upper pressure limit: 80 cmH2O; Pressure lower limit: 0 cmH2O; Oxygen concentration: 21%; Trigger sensitivity: -3 cmH2O; PEEP: 3 cmH2O; Gas source rated working pressure: 0.4 mpa. Test items Alarm sound and alarm lamp test The mute button Test point Inspection standards Advanced alarm: flashing red light, 10-pulse cycle play an alarm sound. Respectively to trigger the high, middle, low-level alarm, observe warning lights and alarm sounds Intermediate warning: flashing yellow light, 3-pulse cycle play an alarm sound. Low-level alarm: yellow light, a single alarm sound of the two pulse. Unplug piping appear sound the alarm, press the mute button; Press the mute button after the sound the alarm disappears, the Screen 120s countdown; 6-21 Tidal volume In standard work status, breathing test device is connected to ventilator inspiratory port in VCV mode, after the output tidal volume is stable, record Vti monitoring values of breathing test equipment and ventilator. Tidal volume settings as the following table: Volume (mL) Inspiratory time(s) Frequency (bpm) Child 20 0.2 60 Adult 500 2 10 Set the value of 20mL, the detection value is 10 ~ 30mL; Set the value of 500mL, the detection value should be 450 ~ 550mL; PEEP The standard working condition, connecting pipes and the simulated lung, in the loop access breath testing equipment, ventilator Set PEEP 20cmH2O, as work, set end-expiratory pressure for follows: 18cmH2O ~ 20cmH2O, other parameters default. Observed 22cmH2O; ventilator monitoring values, values and ventilator settings. Peak velocity Perform the calibration of the flow sensor, disconnect the breath-side piping, and the recorded value of the maximum flow rate of the ventilator. PCV mode, respiratory rate 10 bpm, the upper pressure limit set maximum PEEP was the value of 0 cmH2O with three links breath test Pressure to equipment and ventilator connection, set the control value of the pressure level in the order of 5 levels of cmH2O, 30 cmH2O, 60 cmH2O, breathing PCV machine at the platform when the pressure of the test equipment in the record show the value and set value. FiO2 Exhalation valve 6-22 Set the oxygen concentration in the standard working condition, followed by 21%, 60%, 100%, the value of the oxygen concentration stabilized, record the test equipment and the ventilator oxygen concentration detection value, the ventilator settings value. Boot more than 40 minutes to check exhalation valve roots of the great circle the Inspiratory peak flow rate of 160 to 200 the LPM Set 30 cmH2O, as follows: 27 to 33 cmH2O; Set the oxygen concentration of 21%: 18% ~ 24%; Set the oxygen concentration of 60%: 55% ~ 65%; Set for 100% of the oxygen concentration: 95% ~ 100% (which may appear on the arrow, shall be deemed adopted); Can be heated 6 Disassemble and Functional Testing heating lower half of the heating temperature; NOTE: These items relate to the external battery, the application of optional external battery, or NA. Switched by the AC with standard and optional batteries; Power indicator light display standard and optional the battery indicator, external power supply; two batteries with a charging, the charge indicator lights are bright; Turned on Unplug the AC standard and optional batteries connection at the same time; Power indication, the charge lamp is off, the screen shows the standard and optional batteries, battery powered; Boot after 10 minutes with alone the standard battery, connect the AC; Boot, standard battery can be charged, the display shows the standard battery charging; charging lights; Boot after 10 minutes with alone the optional battery, connect the AC; Boot, optional battery can be charged, the display shows the optional battery charging; charging lights; AC, battery, display check 6-23 7 User Maintenance 7 User Maintenance WARNING: Aeonmed recognizes that cleaning, disinfection, and sterilization practices vary widely among medical institutions. It is not possible to specify or require specific practices that will meet all needs, or to be responsible for the effectiveness of cleaning, sterilization, and other practices carried out in the patient care setting. WARNING: Use a cleaning and disinfection schedule that conforms to your institution’s disinfection and risk-management policies. • Refer to the material safety data as applicable. • Refer to the operation and maintenance manuals of all disinfection equipment. • Do not inhale fumes that may result from any disinfection process. WARNING: Movable and removable parts may clamp or even crush your hand. Use caution when moving or replacing system components. CAUTION: The disposal of environmentally harmful devices (such as batteries and LCD display) must be in accordance with local regulations. WARNING: Use a cleaning and disinfection schedule that conforms to your institution’s disinfection and risk-management policies. • Refer to the material safety data as applicable. • Refer to the operation and maintenance manuals of all disinfection equipment. • Do not inhale fumes that may result from any disinfection process. WARNING: Do not use talc, zinc stearate, calcium carbonate, corn starch or similar material to prevent sticking of the bellows, as these materials may enter the patient’s lungs or airway, causing irritation or injury. CAUTION: To prevent system damage: • Refer to the literature supplied by the manufacturer of the cleaning agent. 7-1 • Never use organic, halogenated or petroleum-based solvents, anesthetic, glass cleaning agents, acetone or other irritant agents. • Never use abrasive agents (i.e. steel wool or silver polish) to clean components. • Keep all liquids away from electronic components. • Prevent liquid from entering the equipment. • All cleaning solutions used must have a pH between 7.0 and 10.5. CAUTION: Never immerse the oxygen sensor or its connector in any type of liquid. • Dispose of the oxygen sensor per the manufacturer’s specification. CAUTION: Do not wash the inner surface of the oxygen sensor. CAUTION: Prior to use after cleaning or disinfecting, power up the system as described in section 6 and follow the on-screen Pre-Use test prompts to perform the Leak Test and Circuit Compliance Test. Discard one time use components after using. Don’t use hard brushes or other sharp tools in cleaning to avoid damage to parts. • Clean components with warm water and light detergent. • Dry the components after rinsing with water. • Check each component when cleaning and replace damaged ones. • After a component is replaced, test the ventilator first prior to putting the ventilator into service. CAUTION: Follow the instructions of detergent manufacturer. Concentrated detergent may hurt some components. Detergent residue may cause spots and cracks, especially in high temperature disinfection. 7.1 Cleaning and Disinfection CAUTION: Before the first use clean, disinfect, and sterilize the ventilator. Disposable components must be disposed in accordance with local regulations. Don’t use hard brushes or other sharp tools in cleaning to avoid damage to parts 7-2 7 User Maintenance 7.1.1 Cleaning and Disinfecting Agents/ Autoclaving Agent • Mild dishwashing detergent • Soapy water with detergent ph between 7.0 and 10.5 • Isopropyl alcohol (70% solution) Classification Detergent Detergent Intermediate level disinfectant • Window cleaning solution (with isopropyl Intermediate level disinfectant alcohol and ammonia) • Sodium hypochloride- (bleach) in water (10% solution) • Hydrogen peroxide (3% solution) Intermediate level disinfectant Intermediate level disinfectant • Gluteraldehyde 2% solution High level disinfectant • Steam autoclaving up to a maximum temperature of 134°C (273°F). High level disinfectant 7.1.2 Cleaning and Disinfection Methods Different parts of the ventilator have their respective cleaning and disinfection methods. The following categories are defined for the parts noted in Table 7-1. The parts need to be cleaned, disinfected and thoroughly dried before reassembly. A: Wipe: If there is a potentially infectious substance on the breathing system, such as blood or secretion, wipe away the substance with disposable cloth using proper disinfectant. Use a soft cloth with a water-soluble detergent or disinfectant wipes. B: Machine washing: Automatic washing with washer and disinfecting with disinfection machine. C: Immersion disinfection: Soak in glutaraldehyde-based formulations of 2%. D: High temperature and pressure disinfection: At 121℃ for 20 minutes minimum, or at 134℃ for 8 minutes minimum. Follow the manufacturer’s instructions for high level disinfection. High-temperature disinfection does not have any cleaning effect. It should only be used on components that have already been cleaned by hand or machine and then thoroughly dried. 7-3 Table 7-1 Cleaning and disinfection methods Part name A B C D √ √ √ Ventilator exterior, including housing, gas supply hoses and power cord √ All components in Figure 7-3 √ CO2 sensor, other breathing circuit parts or accessories Follow the manufacture’s guidelines 7.1.3 Cleaning and Disinfection of Components 7.1.3.1 External Surfaces Using a soft cloth with a water-soluble detergent or disinfectant wipes, clean the housing, gas supply hoses and power cord. 7.1.3.2 Expiratory Module (1) Disassembly Figure 7-1 7-4 Figure 7-2 7 User Maintenance Figure 7-3 To remove the components from expiratory module: e. Press the button(1 in Figure 7-1); f. Pull out the expiratory valve core component (2 in Figure 7-1, Figure 7-2); g. Remove the end cover(1 in Figure 7-3) and the gasket (2 in Figure 7-3); h. Remove the scale board(3 in Figure 7-3)and the diaphragm(4 in Figure 7-3); i. Rotate the expiratory connector (5 in Figure 7-3) clockwise; j. Pull out one-way valve core(6 in Figure 7-3), rubber o-sealing ring(7 in Figure 7-3) and one-way diaphragm(8 in Figure 7-3); k. Rotate the water trap (9 in Figure 7-3), then pull it out. (2) Cleaning a. Wash each component using a mild detergent and water solution. b. Rinse with clean, hot water and allow to thoroughly dry. (3) Disinfection NOTE: Ensure that all the components have been cleaned before disinfecting. Using the Gluteraldehyde disinfection solution, follow the manufacturer’s instructions for high level disinfection and rinsing of all components while adhering to facility policies and procedures. 7-5 All the components can also be high temperature and pressure disinfected. Using an autoclave, follow the manufacturer’s instruction for high level disinfection of all the components while adhering to facility procedures. (4) Assembly Reassemble the components in the reverse order. After installation, please perform a pre-use test and verify all tests passed. 7.2 Regular Maintenance 7.2.1 Maintenance Principles Do not use a faulty machine. Ask an authorized agent of our company to carry out all necessary maintenance tasks. Test the machine after maintenance for normal operation. Every parameter should meet specifications. In order to ensure the reliability of the machine, all maintenance and repair work should be carried out by an authorized agent of our company. In case such person is unavailable, a qualified person with similar maintenance experience is acceptable for the work. CAUTION: Person with no sufficient experience is strictly prohibited to perform maintenance. Only use products provided by Aeonmed to replace damaged items. Verify and test all items ensuring they meet specifications. Contact local service agent of our company in case support is needed. In all cases, maintenance fee is the current component price plus reasonable labor cost, except for those within warranty period. 7.2.2 User Maintenance Minimum maintenance interval Task Daily Check liquid in expiratory module water trap (collected liquid volume cannot be more than half of the bottle) Weekly Calibrate oxygen sensor 7-6 7 User Maintenance Minimum maintenance interval Task 1-3 month(s) Replace air filter Every 6 months Charge and discharge the batteries once (Charge time: at least 3.5h). Calibrate flow and pressure sensors; Every year Calibrate inspiratory valve and expiratory valve (if necessary) Every year or after calibration Replace the O2 sensor(actual life depends on temperature and O2 concentration) When cleaning and installing Check components necessary. and replace or repair when WARNING: If the Ventilator will not be in use for a period of more than 6 months, the internal batteries must be disconnected or removed to prevent possible damage to the equipment or risk to users or service personnel. WARNING: The ventilator must not use, nor be connected to, any anti-static or electrically conductive hoses and tubing. 7.2.3 Service Life of Product/Accessories Service Life is defined as the time that the manufactured device can be expected to be 'serviceable' or supported by our company and the maximum time the device can be used safely. Some items within the device may require maintenance, repair or replacement within this time. Such items will be available from our company for the service life of the product. The calculation of the service life begins at the installation of the product at the customer site. Our company recommends for safe use that each device is replaced after its service life is completed. CAUTION:The service life of the following items is based on normal operating conditions. 7-7 Mask, patient pipeline Sterilize for 20 times at 121℃ Power supply cable, gas supply pipe 8 years Machine 8 years Battery 500 cycles of full charge(The battery from exhausted to full ) 7.3 Maintenance in Operation and Transportation The location of machine should be proper so that it cannot obstruct or be disturbed by medical care personnel. Fix power supply cable well to avoid failure. Use caution not to touch accidental keys on the panel, which may make tidal volume setting wrong. During transportation of the ventilator with or without a patient connected, make sure that the following conditions are fulfilled: Gas cylinders are connected with a sufficient amount of gas, the Battery module is functioning. Follow the hospital guidelines. Use the handles on the Mobile Cart. Transport the bed and the ventilator slowly, and watch the patient connection carefully to see that no pulling or other movement occurs. Be careful not to tip the Mobile Cart when crossing an obstacle such as a doorstep. 7.3.1 Transportation Use care when moving machine within hospital or clinical environment. WARNING: If Control Unit of Ventilator is dropped or damaged during transportation, equipment failure could result in patient injury. WARNING: Tip over hazard – use care when moving Ventilator mounted to Cart as device could tip over leading to injury or damage to the equipment and possible subsequent patient injury. User can carry packaged machine while riding vehicle, plane and train. Impact, severe shock and damp should be avoided during transportation (other conditions should accord with purchase contract), with ambient temperature -20°C~+60°C and relative humidity not more than 95%. In case transportation conditions don’t agree, put the machine in specified operating environment at least 24h before using. 7-8 7 User Maintenance 7.3.2 Storage CAUTION: Do not put ventilator into the shock environment. CAUTION: Do not lay anything heavy on the top. The machine should be stored in a room with temperature -20°C~+60°C and relative humidity –not more than 95% non-condensing, with ventilation and no corrosive gas. CAUTION: The device should be stored in a room that is drafty where no corrosion gas exists. CAUTION: When the storage conditions are beyond the requirements of operational environment, and the storage state is transferred into operation state, the product only can be used after being stored in environment for over 24 hours. 7.4 Fuse Replacement WARNING:Before replacing fuse, first disconnect AC power. Otherwise, it may cause injury or even death. WARNING:When replacing fuse, make sure the new fuse is the same type and size as the old one; otherwise, the ventilator may be damaged. CAUTION: The fuse is a damageable part, and care must be taking when replacing it so no damage occurs. 1. AC Circuit Fuse Fuse replacement steps: • • • • • • Insert screwdriver into the trench (2) of end of fuse box, see Figure 7-4. Pull out fuse holder (1). Remove the fuse (3). Load new fuse. Push the new fuse into the original position gently. Connect AC power, and then start the ventilator to test. 7-9 Figure 7-4 2. DC Power Fuse Fuse replacement steps: • • • • • Insert screwdriver into the trench (1) of end of fuse box, see Figure 7-2. Pull out fuse holder (2). Remove the fuse (3). Load new fuse. Push the new fuse into the original position gently. Figure 7-5 7.5 Battery Maintenance 7.5.1 Battery Specification Battery module - DC12V, 6.6AH, 14.4V lithium-ion battery - Typical charge time: 3.5h - Typical discharge time: 2h When the main power supply voltage is too low or the main power supply fails, two backup batteries (one is necessary, and one is optional) can protect the ventilator. When having a power failure, the ventilator can switch to battery supply automatically, and can normally work without pneumatic power supply failure. The two batteries are usually available for the ventilator working for 4 hours. 7-10 7 User Maintenance 7.5.2 Precautions Charge: When operating with AC power supply on, the system will maintain the battery automatically. Charge time is less than 3.5h. Discharge: The machine is operating on battery. In case of low battery condition, an alarm message “Low battery” will appear, notifying the user to restore AC power supply to charge batteries, otherwise the batteries will be depleted and alarm “Limited Battery Capacity” will be displayed, and eventually the system will be shut down. (For safety reason, manual power-on is required to start the machines again after an automatic shutdown). Before the machine is put into patient use, or disconnecting AC power for transport, or other purposes, check the battery power. If the battery is not fully charged, connect the ventilator to AC power for at least 3.5hours and recharge the battery until the power reaches 80%~100%. 7.5.3 Battery Storage In case the battery is to be stored for a long time, charge it fully prior to storage. To keep the battery power and prolong the battery life, please ensure that the ventilator is connected to the main power. Charge the battery every six months. Actual charging time depends on the storage environment. High humidity and high temperature environments should be avoided for storage. If battery is damaged due to improper maintenance, replace promptly, otherwise liquid leakage may corrupt the machine. Contact the manufacturer when replacing battery. 7.5.4 Battery Replacement Same model battery with CE certification is required. Make sure AC power supply is disconnected before replacing. CAUTION: An authorized Aeonmed service representative can replace battery. If the machine will not be used for long-time, please contact Aeonmed service representatives to disconnect battery. A depleted battery should be disposed in accordance with the local policies. 7-11 7.5.5 Battery Charging and Calibration Use the battery charger supplied by our company to charge or calibrate the battery. After calibrating the battery, the ventilator can read the residual battery capacity accurately. Please charge or calibrate the battery according to the instructions of the battery charger. CAUTION: When ‘low battery’ alarm occurs, charging should be done immediately. The VG70 Ventilator System will shut off in several minutes automatically. 7.6 Oxygen Sensor The oxygen sensor can be used to measure the local oxygen concentration when it is connected to the ventilator or other equipment. The oxygen sensor is suitable for adult and child. 7.6.1 Oxygen Sensor Replacement Disassembly Step 1: Open outward (1), and then remove the cover. Step 2: Pull up the crystal joint of the oxygen sensor, turn the oxygen sensor (3) anticlockwise, and then remove it. 7-12 7 User Maintenance Assembly Inspect the oxygen sensor for damage and replace as necessary. Then reassemble the oxygen sensor. 7.6.2 Oxygen Sensor Calibration For oxygen sensor calibration, refer to section 9.1. 7.6.3 Technical Specifications of Oxygen Sensor Measure range 0 ~ 100 Vol.% Measure accuracy <1% Response time <10s Operating temperature 10℃~ 45 ℃ Pressure range 600hPa ~ 1750hPa Humidity effect -0.03× current humidity Storage temperature -20℃~ 50℃ Drift Air: <1%/month Reproducibility ±1% Type Chemical fuel cell Expect life time 12 months Total system response time <60s Working principle of O2 monitor The O2 monitor surveys and displays the O2 concentration in the patient loop. The oxygen sensor component contains an oxygen sensor, which can produce the voltage proportional to the oxygen partial pressure (concentration) on its detection surface. The oxygen sensor is an electrochemical device (chemical battery). Oxygen expands in this device through a layer of film and oxidizes the base metal electrode. The oxidation process 7-13 produces a current with an amplitude proportional to the oxygen partial pressure indicated by the electrode sensor. The base metal electrode is gradually exhausted during the oxidation process. The voltage of the sensor is influenced by the temperature of the monitoring gas mixture. The surgical thermosensitive resistor of the sensor automatically compensates temperature change in the sensor. The O2 monitor converts the sensor signal into the corresponding oxygen percentage value by using signal processing and circuit analysis. The system displays the value and compares it with the stored alarm limit value. If the value exceeds the threshold, the monitor will alarm. NOTE: After being in a condensing atmosphere, the oxygen sensor shall be stored for more than 24 hours in an environment equivalent to operating humidity. 7.6.4 Oxygen Sensor Maintenance The oxygen sensor should be regularly calibrated. For the calibration interval, refer to section 7.2.2. To improve the life time of the oxygen sensor, when the ventilator is not in use, the oxygen sensor should be avoided contact with the high-concentration oxygen. The oxygen sensor is consumptive, and the period of valid is ordinarily 12 months. So the user should pay attention to the use of the oxygen sensor. When the oxygen sensor fails, replace it. The recommended oxygen sensor is supplied by Aeonmed. WARNING: Do not immerse oxygen sensor in liquid. Do not conduct autoclave or high temperature fumigation on the oxygen sensor. 7.7 Paramagnetic Oxygen Sensor (optional) The paramagnetic oxygen sensor can be used to measure the local oxygen concentration when it is connected to the ventilator or other equipment. The oxygen sensor is suitable for adult and child. 7-14 7 User Maintenance 7.7.1 Paramagnetic Oxygen Sensor Calibration For the paramagnetic oxygen sensor calibration, refer to section 10.1. 7.7.2 Technical Specifications of Paramagnetic Oxygen Sensor Performance Technology Paramagnetic Range 0-100% O2 (with over range -15% O2 to 200% O2) Accuracy (Intrinsic Error) <± 0.2% O2 Linearity <± 0.2% O2 Repeatability <± 0.2% O2 Zero Drift <± 0.4% O2 in first 24 hours, then <± 0.2% O2 /week, then < ± 0.2% O2 /month Response Time (T10 – T90) 8 to 20 seconds dependent on application and filter selection (biological filter on request) Outputs/Controls Signal Output Digital UART or linear mV output (0.5mV or 10mV per % O2) Physical Weight 70g (2.47oz) Dimensions Molex low profile connector: 33.5×30.0×46.1mm (1.32×1.18×1.81”) Diffusion Port Aperture diameter: 15.5mm (0.61”) Sample condition Sample Gas Condition Clean dry gas, free of entrained oil, particulates <3μm, non-condensing Gas Exchange Diffusion Ambient conditions Operation Temperature 5℃ to 50℃ (41℉ to 122℉) Storage Temperature -30℃ to 70℃ (-22℉ to 158℉) Temperature Coefficient Within a range of 0℃ to 50℃: Zero <± 0.5% O2 /10℃, Span: <±0.5% O2 /10℃ Operating Pressure Range ±33kPag (±5psig) 7-15 Ambient Humidity 0 to 95% non-condensing External Power Supply 5V dc, 70mA nominal Power Consumption 350mW 7.8 Disposal This product must not be disposed of with your other waste. Instead, it is your responsibility to dispose of your waste equipment by handing it over to a designated collection point for the recycling of waste electrical and electronic equipment, or by returning it to our company for reprocessing. The separate collection and recycling of your waste equipment at the time of disposal will help to conserve natural resources and ensure that it is recycled in a manner that protects human health and the environment. For more information about where you can drop off your waste equipment for recycling, please contact your local city office, your waste disposal service, or your product distributor or retailer. Correct Disposal of Batteries and O2 Sensors WARNING: Treatment of batteries and O2 sensor: Follow all local regulations with respect to environmental protection when disposing of batteries and O2 sensor. These products contain toxic compounds irrespective of physical condition. They should be disposed of according to local waste management requirements and environmental legislation. They should not be burned since they may give off toxic fumes. Do not throw into fire! Risk of explosion. Do not force open! Danger of bodily injury. 7.9 Manufacturing techniques and materials: For a period of one year from the date of original delivery, the components and assemblies of this product are warranted to be free from defects manufacturing techniques and materials, provided that the same is properly operated under the conditions of normal use and regular maintenance. The warranty period for other parts is three months. Expendable parts are not included. Aeonmed’s obligation under the above warranties is limited to repairing free of charge. 7-16 7 User Maintenance 7.10 Free Obligations: • Aeonmed’s obligation under the above warranties does not include the freight and other fees; • Aeonmed is not responsible for any direct, indirect or failed product or delay which result from improper use, alteration by using unapproved assemblies and maintenance by anyone other than Aeonmed; • This warranty does not apply to the followings: Improper use; Machines without maintenance or machines broken; The label of Aeonmed original serial number or mark is removed or replaced; Other manufacturers’ products. 7.11 Security, reliability and operating condition: Aeonmed is not responsible for the security; reliability and operating condition of this product in case that: • The assemblies are disassembled, extended, altered and/or readjusted • This product is not operated correctly in accordance with the manual instruction. The power supply used or operating environment does not follow the requirements in this manual. 7.12 Return Follow the steps in case the product needs to be returned to Aeonmed: 1、Obtain the rights of return Contact customer service of Aeonmed by informing them of the serial number and type of the product. The number is marked on the surface of the product. Return is unacceptable if the number cannot be identified. Enclose a statement of the number, type and the reason of return. 2、Transportation charges Transportation and insurance charges must be prepaid by the user for transporting the product to Aeonmed for repairing. 7-17 8 Pre-Use Test 8 Pre-Use Test 8.1 Pre-use test procedure After power on, the machine enter Pre-use test. The user can also do all the pre-use test items on Standby screen. Test items on Standby screen are as follows. Test Items Remarks Technical Test After each system has completed its initialization, technical tests will be performed, including: voltage checks at critical points in the circuitry; data collection necessary for system operation; test of communication between sub-systems; tests of measurement circuits and valve control circuits. AC/Battery test This test will verify whether the batteries can supply enough power to operate the ventilator normally. Please follow instructions as displayed. Gas supply test Test will proceed when the hyperbaric oxygen is functional. Oxygen sensor test. This test requires that oxygen supply is available. If oxygen source is not available then a message “oxygen source is inadequate” and the test cannot be carried out. Leak test Internal leakage test. Flow sensor test Flow sensors function and accuracy test. Pressure sensor test Pressure sensors function and accuracy test. Safety valve test Safety valve function and accuracy test. Circuit test Circuit compliance value measurement. CO2 Sensor test Performed if a CO2 module is detected. An Alert shall be posted if the test fails. Methods for testing the function of the alarm system for conditions are specified by IEC60601-2-12. These are to be performed at the user’s discretion. Perform the following procedure to prove operation of the Low MVe and High Airway pressure alarms: 1. Turn the Power switch On. 2. Connect a breathing circuit and test lung to VG70. 8-1 3. Press Start Ventilation and ventilate with default settings, set O2 to 21%. 4. After 5 breaths, observe the MVe reading on the display. 5. Set the MVe low alarm limit to a value greater than the observed MVe reading. 6. Verify that a low level MVe low alarm is present on the 3rd breath. 7. Return the MVe low alarm limit to original setting. 8. After 5 breaths, observe the Ppeak reading on the display. 9. Set the PAW upper alarm limit to a value lower than the observed Ppeak reading. 10. Verify that a low level High Airway Pressure alarm is present after 1 breath and that a high level High Airway Pressure alarm is present at the start of the 4th breath. 11. Return the PAW upper alarm limit to original setting. 12. Turn the Power switch OFF. 8.2 Pre-use test failure analysis If the pre-use test failed, you need to follow the way to troubleshoot. Pre-use test item Possible reason and recommended action if failed: Technical Test See the Technical Test Error Code below. AC/Battery Test Make sure at least one battery is installed and charged. Make sure the AC supply is OK. Gas Supply Test Make sure the oxygen supply is connected, and the pressure is in the spec Oxygen Sensor Test Perform 21% and 100% oxygen sensor test at first, and try again. Make sure the oxygen supply is connected, and the pressure is in the spec. Make sure the oxygen sensor is valid and connected to the main box. Make sure the oxygen auto calibration valve is connected right and no leak. Leak Test Calibrate Inspiratory valve at first, and try again; Make sure machine and connected tube have no leak. Flow Sensor Test Calibrate Inspiratory valve at first, and try again; Make sure machine and connected tube have no leak; Make sure inspiratory flow sensor and expiratory flow sensor work fine. 8-2 8 Pre-Use Test Pre-use test item Possible reason and recommended action if failed: Pressure Sensor Test Calibrate Inspiratory valve at first, and try again; Make sure machine and connected tube have no leak; Make sure inspiratory pressure sensor and expiratory pressure sensor work fine. Safety Valve Test Calibrate Inspiratory valve at first, and try again; Make sure safety valve works fine; Make sure machine and connected tube have no leak; Make sure expiratory pressure sensor works fine. Circuit Test Calibrate Inspiratory valve and Expiratory valve at first, and try again. Make sure machine and connected tube have no leak; Make sure expiratory pressure sensor works fine. CO2 Sensor Test Take off the CO2 sensor from the breath circuit, and put it in the air. Make sure the CO2 sensor has warmed up. The LED indicator in the sensor turns green. Make sure the adapter is installed into the sensor. 8-3 9 Network 9 Network 9.1 Overview The ventilator can export patient monitoring data in HL7 format over a local area network (LAN) using the TCP/IP transport protocol. The data can be sent to a server and then forwarded to an Electronic Medical Records (EMR) system or sent directly to the EMR system. 9.2 What is Exported? Network completes ventilator local IP and port settings, specifies IP (common PC) of node and port number that connect and send data. After doing the above settings can establish a connection to the ventilator. This machine data is sent to the remote destination node. Currently send data by two ways. One is sent cyclically, the period is 1second, and sending waveform data and monitoring values. Another is sent immediately, send alarm information, ventilation parameters, alarm limit settings and so on at the beginning of connection. When the information is changed that send synchronal information. Detailed data communication protocols refer to "HL7 agreement." 9.3 Establishing a Connection Using data transmission functions require checking whether connection is open. Check by following steps: 1) Connect the ventilator and network of purposes PC with network cable. Connection can be achieved by two ways: First, the ventilator access network equipment, such as Hub, switches and so on. Second, connect the ventilator and the destination PC's network card with a network cable. Two connections use ordinary network cable. 2) Ensure that the ventilator set the IP address manually, not automatically obtain IP. USB key board is connected to the ventilator while click two keys Win and Esynchronously, then appear “My Device” interface, press“ control Panel”“Network and Dial-up Connections”“PCI-E100E1”, enter the connection settings interface. Obtaining the IP address is set to: Specify an IP address, and the ventilator is specified an IP address. Set IP address of ventilator and destination PC in the same network segment. Such as 9-1 PC's subnet mask is 255.255.255.0, subnet mask of ventilator also performs the same setting, first three digits setting of ventilator IP address is the same as PC's IP address and ensure that the final one setting is different other hosts on the network segment. Then click “win” button, and select “reboot”, hot restart ventilator. After this operation, ventilator enter the local IP and remote IP and click the Connect button to complete the local IP settings by the ventilator settings →UI interface network interface. You can enter the ventilator control panel to set without keyboard each time 3) Use “Ping” command to verify network connectivity. Use “Ping” command to verify network connectivity on the PC. “Ping” open indicates the network is available, you can click connection button to start data transmission. Click the Connect button, if the ventilator displays IP settings are different the current IP, change interface IP settings for ventilator IP, or retain the current setting. Then start data transmission. 4) The PC starts data receiving test software to check whether the data is sent correctly. The PC starts data receiving test software, input ventilator IP, click “connection”. Ventilator system setting web interface, complete the above 1), 2), 3) operation, click the “connection” that starts data transmission. Received data can be seen by PC-data receiving software. 9.4 Interface Instructions Click “Configurations” to enter the configurations interface. On this page, the user needs to enter the password, as shown in Figure 9-1. Figure 9-1 9-2 9 Network Input the correct password to enter, Click “Site Configuration” to enter the site configuration interface. As shown in Figure 9-2. Figure 9-2 Various parts of the interface are described as follows: Figure 9-3 1) Local IP Address. Each number is from 1 to 255. If the input is outside this range, a message is displayed: “* is invalidated. Please insure the value is between 1 and 255.” If it is not set then display 0.0.0.0. 2) Local subnet mask. If it is not set then display 0.0.0.0. This is generally filled 255.255.255.0 or 255.255.0.0. This set is the same as subnet mask of destination PC. 3) Local port. Ventilator sends data through the port. Receiver gets data from the ventilator through the port. Port 9101 is used by default. It can use from 65535 to 5000 as 9-3 the port number, If the input is outside this range, a message is displayed:” * is invalidate. Please insure the value is between 5000 and 65535.” 4) Remote IP address. It is the IP address of the data receiving end. Once connected, the ventilator only sends data to this address of the host. Usually the IP and ventilator IP is in the same segment. 5) Remote port. It is data receiving end port. Ventilator establishes a connection with the receiver through the port, receiving end receives data through the port. This machine is generally associated with Ventilator port. Port 9101 is used by default. It can use from 65535 to 5000 as the port number, If the input is outside this range, a message is displayed:” * is invalidate. Please insure the value is between 5000 and 65535.” 6) “Connect”. Click this button to start data transmission. A message is displayed: message is displayed on (8) area, while a small icon is displayed in the upper right corner of interface within three seconds. Then all the data will be saved in the ventilator file, when open this interface again, it will display the last settings. 7) “Disconnect”. Click this button to stop the data transfer. 8) Data sharing status. Connected, a message is displayed: “Data Share On”; disconnected, a message is displayed: “Data Share Off”. 9) Digital input keypad. It is used to enter the digital. 9.5 Transmits and Receives Data Data is packaged in HL7 format and transmitted by UDP packets. You can set up UDP listening service to receive data, unpack the data according to the rules of the relevant agreements. Detailed rules refer to "HL7 Protocol." 1) Waveform data. The period of Waveform is 1second. Receiver receives the waveform data and saves into the buffer, and then it is drew on the interface according to the sampling frequency (50ms). Drawing frequency and sampling frequency may be different. It is adjusted according to the number of data in the buffer. Since the network delay and buffer, there will be a delay drawing, the maximum delay is about 7 seconds. 2) Monitoring data. The transmission period is 1 second. Only the network latency is affect monitoring data updates, it is generally not delay in the open network. 3) Alarm information. It sends all current alarm information when connection is established and ventilation is started. It will also send the appropriate information when the new alarm generates or alarm disappears. 4) Mode and ventilation parameters. It sends all current mode and ventilation parameters when connection is established and ventilation is started. It will send the change mode and ventilation parameters settings in the mode transitions. 5) Alarm limit. It sends all current alarm limits when connection is established and 9-4 9 Network ventilation is started. It will send the change value when alarm limit is changed. 9.6 Troubleshooting Right transmission data requires that network settings of ventilator is correct, receiver test software can start normally, and network port of ventilator is installed correctly, and so on. 1) Local network settings of ventilator can refer to 9.3. 2) Receiver test software can move in windows XP and windows 7 system, if it can’t receive data and ventilator and network cable are normally, network configuration information may be incorrect. In this case, you can use the command:”netsh winsock reset” that reset the network configuration and reboot the computer. 3) Ventilator network card. The above case is correctly, the network is still blocked, namely, ping is failure. You should check that ventilator network card is properly installed and connected. Use correct network port which is connected to the ventilator, if you can Unicom network, show the network adapter or interface have problems. Can replace card or connect afresh. 4) The PC's network connection is disconnected if you connect the ventilator and computer with network cable, you should check whether the line is connected properly. Make sure the cable is normal, then should open the ventilator screen, check the card is properly connected. 5) If uld op is fail, make sure “remote IP Address” is the same as PC IP. If the PC uses a wireless connection that can be disconnected, then verify properly connected. 9-5 10 Service Menu 10 Service Menu CAUTION: On “System” of “Main Menu”, “Service” tab is reserved for service only. Press “Service” to enter the “Service” page. On this page, the user needs to enter the password, as shown in Figure 10-1. Figure 10-1 Input the correct password to enter, There are six choices on this page: Calibration, Event/Alarm Log, Machine Information, Language, Test Page and Update. The default page is Calibration. See Figure 10-2. Figure 10-2 10-1 10.1 Calibration 10.1.1Calibration operation guidance The calibration choices include: 1)Pressure Sensor Calibration, 2) Flow Sensor Calibration, 3) O2 Sensor Calibration, 4)CO2 Sensor Calibration, 5) Inspiratory valve Calibration, 6) Expiratory Valve Calibration, 7) Atmospheric Sensor Calibration, 8) Touch Screen Calibration, 9) Leak Test and 10) Breath Circuit test, as shown in Figure 10-2. 1) Pressure Sensor Calibration: Press “Pressure Sensor” to enter the calibration interface. A message is displayed: “This step is to zero the pressure sensor. Please remove the breathing circuit from the ventilator before calibration.” A legend is displayed as well as shown in Figure 10-3. Figure 10-3 Press “Start” to start pressure sensor calibration. A progress bar and a message “Calibration in progress. Please Wait” will be displayed as shown in Figure 10-4. NOTE: During this period no other operation can be performed. Pressing other areas will have no response. 10-2 10 Service Menu Figure 10-4 2) Flow Sensor Calibration: Press “flow sensor” to enter the interface. A message is displayed: “This step is to calibrate the flow sensor. Please connect the insp. Port and Exp. Port directly with a tube” as shown in Figure 10-5. Figure 10-5 Press the “Start” button to start flow sensor calibration, the remaining procedure is the same as the pressure sensor calibration. 3) O2 Sensor Calibration: Press “O2 sensor” to enter the interface. A message displayed: “Please verify that the oxygen source are connected correctly. Verify that the gas inlet pressure is within specification.” A legend will also be shown. There are two keys below the legend: “Start 21%” and “Start 100%”. Choose the needed one and Press, as shown in Figure 10-6. The remaining procedure is the same as described above. 10-3 Figure 10-6 4) CO2 Sensor Calibration: Press “CO2 Sensor” to enter the interface, as shown in Figure 10-7. A message displayed: “Disconnect the CO2 sensor with the adapter from breathing circuit and ensure it is in ambient air. Wait 1 minute for warm up after the unit is powered on or after installing an airway adapter. Press “Zero” when the State-Area turns green”. Please follow the prompt message to calibrate. Figure 10-7 5) Inspiratory Valve Calibration: Press “inspiratory valve” to enter the calibration interface. A message is displayed: “This step is to calibrate the inspiratory valve. Please connect the Insp. Port and Exp. Port directly with a tube.” as shown in Figure 10-8. To get a precise calibration, please connect patient tubes with a humidifier before inspiratory valve calibration. Click the “Start” button to start inspiratory valve calibration, the remaining procedure is the same as the pressure sensor calibration. 10-4 10 Service Menu Figure 10-8 6) Expiratory Valve Calibration: Press “Expiratory valve” to enter the calibration interface. A message is displayed: “This step is to calibrate the expiratory valve. Please connect patient circuit and test lung before calibration.” as shown in Figure 10-9. Click the “Start” button to start expiratory valve calibration, the remaining procedure is the same as the pressure sensor calibration. Figure 10-9 7) Touch Screen Calibration: Press “Touch screen” to enter the calibration interface. A message is displayed: “This step is to calibrate the touch screen. The ventilator’s screen will disappear during the calibration. Please follow the instruction in the calibration program.” as shown in Figure 10-10. CAUTION: Please calibrate the touch screen periodically or when it works abnormally. 10-5 Figure 10-10 Press the “Start” key to start, the remaining procedure is as described in steps above. 8) Leakage Test Calibration: Press “Leakage Test” to enter the test interface. A message is displayed: “This step is to test the internal leakage of ventilator. Please connect the Insp. Port and Exp. Port directly with a tube”, as shown in Figure 10-11. Figure 10-11 Press “Start” to start the leakage test. A progress bar and a message “Test in progress, please wait” will be displayed as shown in Figure 10-12. 10-6 10 Service Menu Figure 10-12 9) Breath Circuit Test Calibration: Press “Breath Circuit Test” to enter the test interface. A message is displayed: “This step is to test the compliance and leakage of breathing circuit. Please connect the patient circuit to the T-piece, and plug up the patient end of the T-piece”, as shown in Figure 10-13. Before starting the test, ensure the patient circuit has been connected to the T-piece and the patient end of the T-piece has been plugged up. Press the “Start” button to start the breath circuit test. Figure 10-13 10-7 10.1.2Calibration failure analysis Calibration items Possible reason and recommended action if failed: Pressure Sensor Make sure inspiratory pressure sensor and expiratory pressure sensor work fine; Make sure no gas flow pass inspiratory pressure sensor and expiratory pressure sensor. Flow Sensor Make sure inspiratory flow sensor and expiratory flow sensor work fine; Make sure no gas flow pass inspiratory flow sensor and expiratory flow sensor. O2 Sensor Calibrate Inspiratory valve and Expiratory valve at first, and try again; Make sure the oxygen supply is connected, and the pressure is in the spec; Make sure the oxygen sensor is valid and connected to the main box. CO2 Sensor See Section 8.2 Inspiratory Valve Make sure the oxygen supply is connected and work normal; Make sure machine and connected tube have no leak; Make sure inspiratory flow sensor and expiratory flow sensor work fine. Make sure inspiratory valve works fine. Expiratory Valve Make sure the oxygen supply is connected and work normal; Make sure machine and connected tube and lung have no leak; Make sure inspiratory pressure sensor and expiratory pressure sensor work fine; Make sure inspiratory valve and expiratory valve work fine. Touch Screen Trying the fewest test points calibration (4 points). Make sure press the center of each test point (the red region). Make sure the plastic frame of screen doesn’t constrict the touching area of touch screen. 10-8 10 Service Menu 10.2 Event/Alarm Log Press “Event/Alarm Log” to enter “Event/Alarm Log” page, as shown in Figure 10-14. Figure 10-14 The middle area of this page is the message area. It can store up to 1000 messages, including event messages and alarm messages. All messages will be listed in time sequence. The top is the latest event or alarm message, and the bottom is the oldest. Use the scroll bar to check all the messages. An asterisk (*) in front of an alarm message indicates that alarm message was not displayed in the alarm message area. As shown in Figure 10-15. The event/alarm log records all alarms and most actions. Figure 10-15 Below is the message area of the Settings area resulting from highlighting an alarm. All settings will be given here, as shown in Figure 10-16. Figure 10-16 NOTE: Alarm/Event Log data is retained during a power interruption and can be viewed when power returns. 10-9 10.3 Machine Information Press the “Machine information” key to enter the Machine information page, this area includes the following information, as shown in Figure 10-17. 1. Software Version: a. UI b. BDU c. Power Supply 2. Runtime Hours 3. O2 Source Pressure 4. Atmospheric Pressure Figure 10-17 10.4 Language Press “Language” to enter the language screen. English and other languages are available for the user to choose. 10.5 Test Page Press “Test Page” to enter the test page. There are five choices on this page: Demo, Vlt. Monitor, Schematic, Cali. Data, Service Timer, Error Code and PT100 Cali. as shown in Figure 10-18. 10-10 10 Service Menu Figure 10-18 10.5.1Demo Press “Demo” to enter the demo setting page, as shown in Figure 10-19. In this page, the operator can turn on or turn off the demo mode, and test the broken pixel of the screen. Figure 10-19 10.5.2Vlt. Monitor Press “Vlt. Monitor” to enter the vlt. monitor page, as shown in Figure 10-20. This page displays all the BDU and PS circuit monitoring value. 10-11 Figure 10-20 10.5.3Schematic Press “Schematic” to enter the schematic page. The schematic is as shown in Figure 10-21. In this page, the operator can control all valves and read all sensors state. The valves (#1 to #9) and the Exp. Valve Heater shall have two states: on and OFF; The Turbine voltage range: 0-5, the units: V, the resolution: 1; The Insp. Valve range: 0-180, the units: LPM, the resolution: 0.5; The IP Valve range: 0-100, the units: cmH2O, the resolution: 1. Figure 10-21 10-12 10 Service Menu 10.5.4Cali. Data Press “Cali. Data” to enter the cali. data page, as shown in Figure 10-22. This page displays the calibration data of Inspiration /Expiration Sensor. Press “Refresh” button to update the calibration data and the zero AD value of the sensor. Press “Manual Cali.” button to display the manual calibration page; in the page, the operator can manually calibration the Insp/Exp sensor. Figure 10-22 10.5.5Service Timer Press “Service Timer” to enter the service timer page, as shown in Figure 10-23. This page displays the service time about the ventilator, the turbine runtime, and the int./opt. battery charging/discharging circle count. Figure 10-23 10-13 10.5.6 Error Code Press “Error Code” to enter the Error Code interface, as shown in Figure 10-24. Figure 10-24 10.5.7 PT100 Cali. Press “PT100 Cali.” to enter the PT100 calibration interface, as shown in Figure 10-25. Figure 10-25 10-14 10 Service Menu The calibration steps are as follows. Step 1: Press “Stop Heater” and wait for 40min to make the expiration valve to cool to ambient temperature. Then measure and record the temperature of the expiration valve. Step 2: Press “ ” turning the key background color to yellow, adjust the value equal to the temperature recorded at Step 1, then read the value “ value in Textbox 1. Step 3: Press “ ” and record the ” turning the key background color to yellow, adjust the value equal to the setting temperature, then read the value “ ” and record the value in Textbox 3.(The setting temperature: the highest temperature of the expiration value heating, default: 35℃. ) Step 4: In turn, press “Write”, “Read”, “Set Point”, “Read Point”, then determine that the value of Textbox 2 is equal to the value of Textbox 1 and the value of Textbox 4 is equal to the value of Textbox 3. If same, the calibration is finished; if not, carry out Step 5. Step 5: Repeat the above steps. 10.6 Update Press “Update” to enter the update page, as shown in Figure 10-26. In this page, the operator can update the software. Figure 10-26 10-15 10.7 Optional Press “Optional” to enter the optional page, as shown in Figure 10-27. In this page, the operator can choose configurable functions. Figure 10-27 10-16 11 Troubleshooting 11 Troubleshooting 11.1 Technical Error 11.1.1Technical Test Error Code The error code is made up by 8 digits hexadecimal numbers, which are four byte using binary system. The highest two bytes are internal AD test result flag, the lowest byte are external AD test result flag. The meaning of each bit is shown in the below table. External AD##(0xF FFF00xx) Bit 0 Bit 1 Test Point Possible Reason and Recommended Action Zero point of expiratory flow rate 1. Remove the test lung and breath circuits, perform the test again. 2. Don’s start PUT too quickly after quit from ventilation. 3. Calibrate the pressure, flow rate sensor, Insp. valve and Exp valve. 4. Proportional solenoid valve can’t close completely. Replace proportional solenoid valve. 5. Replace expiratory flow rate sensor. Zero point of inspiratory flow rate 1. Remove the test lung, connect Insp. port and Exp. port directly with a tube. 2. Don’s start PUT too quickly after quit from ventilation. 3. Calibrate the pressure, flow rate sensor, Insp. valve and Exp valve. 4. Proportional solenoid valve can’t close completely. Replace proportional solenoid valve. 5. Replace mother board. 1. 2. Bit 2 Inspiratory temperature 3. 4. Check the cable between the mother board to the temperature sensor The interface IC on the mother board is broken. Replace mother board. The input port of external AD chip on the mother board is broken. Replace mother board. Replace temperature sensor. 11-1 Bit 3 -- -- Zero point of expiratory pressure 1. Remove the test lung, connect Insp. port and Exp. port directly with a tube. 2. Don’s start PUT too quickly after quit from ventilation. 3. Calibrate the pressure, flow rate sensor, Insp. valve and Exp valve. 4. Proportional solenoid valve can’t close completely. Replace proportional solenoid valve. 5. The amplifier on the mother board is broken. Replace mother board. 6. The input port of external AD chip on the mother board is broken. Replace mother board. 7. The sensor is broken. Replace mother board. Zero point of inspiratory pressure 1. Remove the test lung, connect Insp. port and Exp. port directly with a tube. 2. Don’s start PUT too quickly after quit from ventilation. 3. Calibrate the pressure, flow rate sensor, Insp. valve and Exp valve. 4. Proportional solenoid valve can’t close completely. Replace proportional solenoid valve. 5. The amplifier on the mother board is broken. Replace mother board. 6. The input port of external AD chip on the mother board is broken. Replace mother board. 7. The sensor is broken. Replace mother board. Bit 6 Barometric pressure 1. Proportional solenoid valve can’t close completely. Replace proportional solenoid valve. 2. The amplifier on the mother board is broken. Replace mother board. 3. The input port of external AD chip on the mother board is broken. Replace mother board. 4. The sensor is broken. Replace mother board. Bit 7 -- -- Internal AD(0xxxx x00FF) -- -- Bit 15 -- -- Bit 16 -- -- Bit 17 -- -- Bit 18 -- -- Bit 4 Bit 5 11-2 11 Troubleshooting Bit 19 Bit 20 Expiratory valve AD 1. Poor connection between the mother board and daughter board. Re-plug the daughter board. 2. The driver chip of expiratory valve on the mother board is broken. Replace mother board. 3. The daughter board is broken. Replace daughter board. Inspiratory valve AD 1. Poor connection between the mother board and daughter board. Re-plug the daughter board. 2. The daughter board is broken. Replace daughter board. Bit 21 -- -- Bit 22 -- -- Bit 23 -- -- Bit 24 -- -- Bit 25 -- -- Bit 26 -- -- Bit 27 2.5V reference voltage -- Bit 28 -- -- Bit 29 -- -- Bit 30 -- -- Bit 31 -- -- 1. Error Codes in the Log Error Code Test Point Possible Reason and Recommended Action 1000 T12 DC The A5V out is out of range. Replace PS board. 1001 T1 DC The W24V output is out of range. Replace switch PS. 1002 T13 DC The P12V output is out of range. Replace PS board. 1004 T19 DC The N24V output is out of range. Replace PS board. 1008 T24 DC PS board failure. Replace PS board. 1009 T14 DC The A12V output is out of range. Replace PS board. 1013 -- During expiratory valve heating the temperature is out of range. Replace PS board. 2000 T31 DC During expiratory valve heating the output voltage is out of range. Replace PS board. 2001 T30 DC During expiratory valve heating the output current is out of range. Replace PS board. 3006 -- PS board communication failure. Replace PS board. 3005 -- BDU communication failure. Replace core board. 3002 -- Software mismatch failure. Update software. 11-3 11.1.2Other Errors Alarm sound from buzzer in the PS board 1) Communication failure between UI to PS board 2) No AC and battery connected Check if the UI are working. Make sure the cable linking to the PS board is OK. Replace power supply board. 11.1.3Service Tools Service tools are listed underside: Sequence number Name Quantity Remark 1 Metric hex driver set One set ---- 2 #2cross screwdriver One entries Staff width is about 5mm 3 Multi-meter One block ---- 4 VG70 checkout frock and its attachments for ESD (Electro-Static Discharge) protection) One desk If anyone of the electric machine, electric machine driver and the main board is replaced 5 Breath pipeline cover bag One set ---- 6 Simulated lung One entries ---- 11-4 12 Appendix A 12 Appendix A Contact & Ordering Information How to Call for Service This service manual provides procedures for testing and maintaining the Ventilator VG70. It is not intended to be a complete maintenance document; therefore, it contains no detailed disassembly or reassembly instructions. Refer any repairs or adjustments that exceed the scope of this manual to the Service Center of Aeonmed by calling 800-810-8333. This manual contains proprietary information. It is intended for use only by individuals qualified in the installation and maintenance of the Aeonmed ventilator. Receipt, purchase, or possession of this document in no way confers or transfers any other rights for the use of this information. Disclosure or reproduction of the enclosed, without the written permission of Aeonmed is prohibited. This manual is intended for use only by technicians who have successfully completed Aeonmed training on this product. Aeonmed believes the information herein is accurate but accepts no responsibility for errors, omissions, or misrepresentation. 12-1 13 Appendix B 13 Appendix B Diagrams and Schematics Pneumatic diagram 13-1 14 Appendix C 14 Appendix C Specifications System Operating Conditions Operating Temperature Range: +5 to +40 ℃ Relative Humidity: 5 to 95% non-condensing Atmospheric Pressure: 700 to 1060 hPa Non-operating Conditions Storage Temperature Range: -20 to +60 ℃ Storage Relative Humidity: ≤95% non-condensing Storage Atmospheric Pressure: 700 to 1060 hPa Power Supply Power Supply, Automatic Range Selection Battery Backup (Standard and Optional) Maximum Power Consumption 100-240 +/- 10% VAC 50/60 Hz Two rechargeable battery modules, 12 V, 6.6 Ah each. Recharge time approximately 3.5hours. Battery backup time of 120minutes minimum with only standard internal battery. CAUTION: The Power Supply should meet the above specifications. Ventilator General Dimensions: User Interface: 350 wide x 55 deep x 244 high (mm) Ventilation Delivery Unit: 322 wide x 375 deep x 366 high (mm) System with Cart (optional): 547 wide x 675 deep x 950 high (mm) Weight: Total: 40kg (Approximate) User Interface: 2.5kg Ventilation Delivery Unit: 12.5kg 14-1 Cart: 25kg Trigger Method: Flow and Pressure Maximum Limited pressure: 80 cmH2O Maximum Working pressure: 80cmH2O Gas Supply Supplied gases must be free of water, oil and particles Inlet Gas Pressure O2: 280kPa to 600kPa (41– 87PSI) Connection Standards Available DISS, NIST Patient System Connectors Male 22 mm Conical Fittings in accordance with ISO 5356-1 User Interface Attaches to the cart, a rail, or other mounting system. Standard Conditions Specifications Error ranges in this document assume the following standard conditions: Ambient pressure: 101.3 kPa (1 atmosphere) Room temperature: 20 ℃ Dry gases in patient system Inlet pressure: 345 kPa (50 PSI) Inspiratory Channel Pressure Drop Maximum 5 cmH2O at a flow of 60 liters/min without CO2 airway adapter. Compliance Maximum 2 ml/cmH2O (with Fisher &Paykel MR 810 humidifier and reusable silicone adult patient circuit) Gas Delivery System Microprocessor controlled valves Gas Delivery Device Flow Range: 14-2 14 Appendix C 1 to 180 liters/min. (Adult) 0 to 60 liters/min. (Child) Maximum Pressure Setting: 70 cmH2O NIV Max leakage compensation level Adult: 60liters/min Child: 30liters/min Expiratory Channel Pressure Drop (Resistance) Maximum 5 cmH2O at a flow of 60 liters/min without CO2 airway adapter. Compliance Maximum 2 mL/cmH2O (with Fisher & Paykel MR 810 humidifier and reusable silicone adult patient circuit) PEEP Regulation Microprocessor controlled valve PEEP Setting Range: 0 – 35 cmH2O Expiratory Flow Measurements Range: 0 – 180 liters/min. Monitoring Expiratory Minute Volume Range: 0 – 60 liters/min. Accuracy: +/- 1 LPM or +/- 15% of measured value (whichever is greater) Resolution: 0.1 liters > 1 liter/min, 0.001 liters < 1 liter/min Expiratory Tidal Volume Range: 0 – 4000 mL Adult Accuracy: +/- 25 mL or ±15% of the measured value (whichever is greater) Child Accuracy: +/- 10 mL or ±10% of the measured value (whichever is greater) Resolution: 1 mL O2 Concentration Range: 18 – 100% Accuracy: +/- 3 vol. % 14-3 Resolution: 1% Airway Pressure Range: -20 to 80 cmH2O Accuracy: ± (2 cmH2O + 4% of reading) Resolution: 1 cmH2O 14-4 15 Appendix D 15 Appendix D Spare parts and configurations Spare parts list: No. Replacement period Remarks Code Description 1 122007310 NBP mother board As needed —— 2 122007306 NBP power supply board As needed —— 3 122007308 NBP main control board As needed —— 4 122007309 NBP core board As needed —— 5 122007311 NBP interface board As needed —— 6 122005527 Gas supply pressure board As needed —— 7 122007482 Encoder knob component As needed —— 8 122007483 Mute button component As needed —— 9 122007304 NBP display interface board As needed —— 10 122007303 Common display platform core board As needed —— 11 122007305 Common display platform mother board As needed —— 12 122007485 Alarm lamp component As needed —— 13 122007486 NBP indicate lamp component As needed —— 14 122010401 Differential pressure transmitter board As needed —— 15 210003677 Turbine As needed —— 16 210002403 Flower sensor TSI 840201 As needed —— 17 122007463 Inspiration control valve As needed —— 18 240000089 Check valve As needed —— 19 240000252 Electromagnetic valve SY114-6G As needed —— 20 240001020 Mini-electromagnetic valve N332/2B As needed —— 21 210003856 High rupture fuse T250V/3.15A As needed —— 15-1 Replacement period Remarks No. Code Description 22 210003676 Brushless motor driver As needed —— 23 210003693 Touch panel AMT9542 As needed —— 24 210003675 LCD As needed —— 25 122007471 Exhalation valve components As needed —— 26 122007890 Exhalation valve core assemble As needed —— 27 210003491 Switch power supply SNP-G169-M As needed —— 28 210003678 Switch power supply As needed 29 122007322 Fan cable As needed —— Consumables list: No. Code Description Replacement Period Remarks 1 130000231 Valve 1 years or as needed —— 2 130001347 One-way diaphragm 1 years or as needed —— 3 130009651 Water trap 2 years or as needed —— 4 130010423 Fan filter cotton 2 years or as needed —— 5 210001975 O2 sensor 1 years or as needed —— 6 230000135 Filter element 2 years or as needed —— 7 210003734 Lithium-ion battery RC2024 2 years or as needed —— 8 130004358 Filter element 3 months or as needed —— 9 130003930 Filter 2 years or as needed —— 15-2 This manual No.: 130015826 CE mark in this manual apply only to product with CE mark. Directive 93/42/EEC concerning Medical Devices Edition 01.04 May. 2018