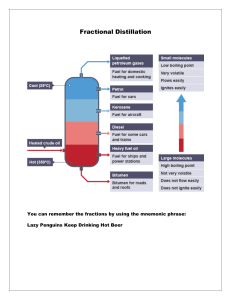
Distillation Techniques Distillation ➢Theory • What is distillation used for? • For what type of compounds? • What is the basic principle? ➢Experiment • Procedure • Equipment 2 Distillation - Theory • Distillation is a technique used to separate or purify liquids based on boiling points. 1. Simple distillation 2. Fractional distillation Simple distillation 3. Vacuum distillation 4. Steam distillation 3 1. Simple distillation - Theory Simple distillation can be used when • Two components have very different boiling points Or • One component is at fairly small amount small difference in bp large difference in bp 4 1. Simple distillation - process • The heated liquid vaporizes and rises upward, past the thermometer into the condenser. • The vapor is condensed into liquid and collected in the receiving flask. 5 1. Simple distillation apparatus Thermometer Boiling stones Distillate 6 1. Simple distillation apparatus 7 1. Simple distillation apparatus Thermometer Thermometer adapter Water out Water in Distilling head Distilling flask Hot plate Condenser Receiving flask 8 9 chem.libretexts.org 2. Fractional distillation Fractional distillation can be used when • Difference in boiling points is not large Or • Highly pure compound is needed 10 2. Fractional distillation apparatus Fractional distillation Simple distillation Fractionating column (Vigreux column) 11 2. Fractional distillation apparatus Fractionating column Fractionating column Graduated cylinder Ring stand 12 2. Fractional distillation apparatus Many vaporization-condensation cycles → better separation Fractionating column 13 Explain the process of separating acetone (bp 56 °C) and toluene (bp 110 °C) 14 chem.libretexts.org